RX 821002 hydrochlorideCAS# 109544-45-8 |
- I-BET-762
Catalog No.:BCC4474
CAS No.:1260907-17-2
- Bromodomain Inhibitor, (+)-JQ1
Catalog No.:BCC1132
CAS No.:1268524-70-4
- I-BET151 (GSK1210151A)
Catalog No.:BCC4476
CAS No.:1300031-49-5
- GSK1324726A
Catalog No.:BCC4038
CAS No.:1300031-52-0
- PFI-1 (PF-6405761)
Catalog No.:BCC2225
CAS No.:1403764-72-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

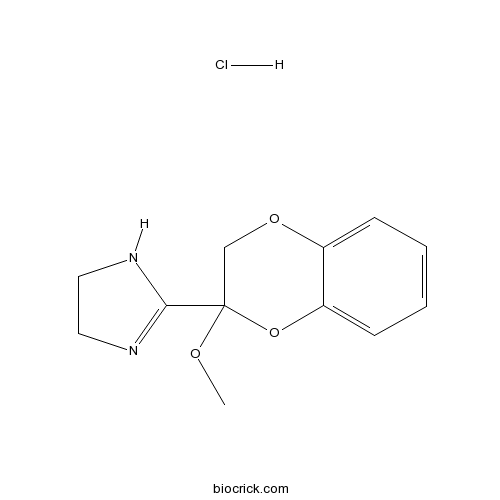
| Cas No. | 109544-45-8 | SDF | Download SDF |
| PubChem ID | 11957683 | Appearance | Powder |
| Formula | C12H15ClN2O3 | M.Wt | 270.72 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water and to 100 mM in DMSO | ||
| Chemical Name | 2-(3-methoxy-2H-1,4-benzodioxin-3-yl)-4,5-dihydro-1H-imidazole;hydrochloride | ||
| SMILES | COC1(COC2=CC=CC=C2O1)C3=NCCN3.Cl | ||
| Standard InChIKey | IMPOOMVZVWKSAP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H14N2O3.ClH/c1-15-12(11-13-6-7-14-11)8-16-9-4-2-3-5-10(9)17-12;/h2-5H,6-8H2,1H3,(H,13,14);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, selective α2-adrenoceptor antagonist with very low affinity for imidazoline sites. Displays selectivity for the α2D over the α2A subtypes (pKd values are 9.7 and 8.2 respectively). |

RX 821002 hydrochloride Dilution Calculator

RX 821002 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6939 mL | 18.4693 mL | 36.9385 mL | 73.8771 mL | 92.3463 mL |
| 5 mM | 0.7388 mL | 3.6939 mL | 7.3877 mL | 14.7754 mL | 18.4693 mL |
| 10 mM | 0.3694 mL | 1.8469 mL | 3.6939 mL | 7.3877 mL | 9.2346 mL |
| 50 mM | 0.0739 mL | 0.3694 mL | 0.7388 mL | 1.4775 mL | 1.8469 mL |
| 100 mM | 0.0369 mL | 0.1847 mL | 0.3694 mL | 0.7388 mL | 0.9235 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- CCT137690
Catalog No.:BCC2188
CAS No.:1095382-05-0
- ARQ 621
Catalog No.:BCC6534
CAS No.:1095253-39-6
- Rac1 Inhibitor W56
Catalog No.:BCC5886
CAS No.:1095179-01-3
- PF-04449913
Catalog No.:BCC5154
CAS No.:1095173-27-5
- JNJ-31020028
Catalog No.:BCC5516
CAS No.:1094873-14-9
- 3'-Methyl-4-O-methylhelichrysetin
Catalog No.:BCN4062
CAS No.:109471-13-8
- BIX 02189
Catalog No.:BCC2549
CAS No.:1094614-85-3
- BIX 02188
Catalog No.:BCC2550
CAS No.:1094614-84-2
- Fmoc-His(Trt)-OPfp
Catalog No.:BCC3502
CAS No.:109434-24-4
- Fmoc-Orn(Boc)-OH
Catalog No.:BCC3533
CAS No.:109425-55-0
- Fmoc-His(Trt)-OH
Catalog No.:BCC3501
CAS No.:109425-51-6
- SCH-1473759
Catalog No.:BCC1934
CAS No.:1094069-99-4
- Tacrolimus monohydrate
Catalog No.:BCC5284
CAS No.:109581-93-3
- Pinocembrin 7-acetate
Catalog No.:BCN5887
CAS No.:109592-60-1
- Topazolin
Catalog No.:BCN6833
CAS No.:109605-79-0
- Neocryptotanshinone
Catalog No.:BCN3158
CAS No.:109664-02-0
- SPK-601
Catalog No.:BCC1961
CAS No.:1096687-52-3
- MLN 2480
Catalog No.:BCC1771
CAS No.:1096708-71-2
- Murraol
Catalog No.:BCN5888
CAS No.:109741-38-0
- cis-Dehydroosthol
Catalog No.:BCN4735
CAS No.:109741-40-4
- 8 beta-(4-Acetoxy-5-hydroxytigloyloxy)costunolide
Catalog No.:BCN7123
CAS No.:109770-86-7
- Homopahutoxin
Catalog No.:BCN1812
CAS No.:109777-68-6
- 9R-10alpha-Hydroxyepigambogic acid
Catalog No.:BCN3079
CAS No.:1097882-33-1
- ASP3026
Catalog No.:BCC1372
CAS No.:1097917-15-1
Antagonists that differentiate between alpha 2A-and alpha 2D-adrenoceptors.[Pubmed:8692278]
Naunyn Schmiedebergs Arch Pharmacol. 1996 Feb;353(3):245-9.
Four antagonists were examined for their ability to differentiate alpha 2A-from the orthologous alpha 2D-adrenoceptors. The antagonists were (2S,12bS)1',3'-dimethylspiro(1,3,4,5',6,6',7,12b-octah ydro-2H- benzo[b]furo[2,3-a]quinolizine)-2,4'-pyrimidin-2'-one (MK912), 2-[2-(methoxy-1,4-benzodioxanyl)imidazoline (RX 821002), efaroxan and benoxathian. The alpha 2-autoreceptors in rabbit brain cortex were chosen as alpha 2A-and the alpha 2-autoreceptors in guinea-pig brain cortex as alpha 2D-adrenoceptors. Slices of the brain cortex were preincubated with 3H-noradrenaline and then superfused and stimulated electrically by brief pulse trains (4 pulses, 100 Hz) that led to little, if any, alpha 2-autoinhibition. 5-Bromo-6-(2-imidazolin-2-ylamino)-quinoxaline (UK 14,304) was used as an alpha 2-adrenoceptor agonist. UK 14, 304 decreased the stimulation-evoked overflow of tritium. The antagonists shifted the concentration-inhibition curve of UK 14, 304 to the right in an apparently competitive manner. Dissociation constants of the antagonists were calculated from the shifts. MK 912, RX 821002 and efaroxan had markedly higher affinity for (guinea-pig) alpha 2D-adrenoceptors (pKd values 10.0, 9.7 and 9.1, respectively) than for (rabbit) alpha 2A-adrenoceptors (pKd 8.9, 8.2 and 7.6, respectively). Benoxathian had higher affinity for alpha 2A-(pKd 7.4) than for alpha 2D-adrenoceptors (pKd 6.9). Ratios calculated from the Kd values of the four compounds differentiated between alpha 2A and alpha 2D up to 100 fold. It is concluded that MK 912, RX 821002, efaroxan and benoxathian are antagonists with high power to differentiate alpha 2A-from alpha 2D-adrenoceptors.
Does [3H]2-methoxy-idazoxan (RX 821002) detect more alpha-2-adrenoceptor agonist high-affinity sites than [3H]rauwolscine? A comparison of nine tissues and cell lines.[Pubmed:7791100]
J Pharmacol Exp Ther. 1995 Jun;273(3):1287-94.
The authors compared [3H]2-methoxy-idazoxan (RX 821002) and [3H]rauwolscine binding in rat cerebral cortex, spleen and kidney; guinea pig kidney; porcine kidney; human kidney and platelets and HEL and NG 108-15 cells. [3H]RX 821002 had less nonspecific binding and higher affinity than [3H]rauwolscine in most models. Although both ligands detected similar alpha-2 adrenoceptor numbers in rat, porcine and human kidney and in NG 108-15 cells in saturation experiments, [3H]RX 821002 detected more alpha-2 adrenoceptors than [3H]rauwolscine in rat cerebral cortex and spleen, guinea pig kidney, human platelets and HEL cells. These differences were seen in Tris and in glycylglycine buffer regardless of whether EDTA, MgCl2, MgCl2 plus GTP or GTP plus NaCl was added to the former and were not explained by additional labeling of serotonin or dopamine receptors or nonadrenergic sites; in contrast, [3H]rauwolscine also labeled nonadrenergic sites in porcine kidney. In prazosin competition experiments, both ligands differentially recognized alpha-2-adrenoceptor subtypes but this could not account for the observed differences in detected receptor numbers. In epinephrine competition experiments, both ligands labeled similar numbers of agonist low affinity sites in all models; [3H]RX 821002, however, labeled more agonist high-affinity sites than [3H]rauwolscine did in models in which it detected a greater total number of receptors. It was concluded that [3H]RX 821002 is a more suitable ligand for the detection of alpha-2 adrenoceptor than [3H]rauwolscine because of less nonspecific binding, higher affinity and greater specificity for alpha-2 adrenoceptors; moreover, [3H]rauwolscine appears not to detect all agonist high-affinity sites of alpha-2 adrenoceptors.
Characterization of [3H]RX821002 binding to alpha-2 adrenergic receptor subtypes.[Pubmed:7908054]
J Pharmacol Exp Ther. 1994 Mar;268(3):1362-7.
Alpha-2 adrenergic receptors have been divided into four pharmacological subtypes based on their differences in affinity for several drugs. Previous studies showed that [3H]RX821002 has a high affinity for the alpha-2A subtype. The current study characterized the binding properties of [3H]RX821002 [2-(2-methoxy-1,4- benzodioxan-2yl)-2-imidazoline] to the alpha-2A receptor in CHO-C10 cells, alpha-2B in neonatal rat lung, alpha-2C in OK cells and alpha-2D in bovine pineal gland. Membrane binding studies of [3H]RX821002 were done in 25 mM glycylglycine buffer at room temperature. The nonspecific binding rates at the KD concentration were 4.9%, 20%, 14% and 8.3% of the total for CHO-C10, neonatal rat lung, OK cells and bovine pineal, respectively, which were determined by adding 100 microM norepinephrine. Saturation curves indicate that [3H]RX821002 has a high affinity for all alpha-2 adrenergic subtypes. The KD values were 0.29, 1.05, 0.37 and 0.19 nM for CHO-C10, neonatal rat lung, OK cells and bovine pineal, respectively. [3H]Rauwolscine has affinities of 0.34, 0.55 and 0.24 nM for the alpha-2A, -2B and -2C subtypes. By contrast, [3H]rauwolscine has a much lower affinity for alpha-2D subtype with a KD value of 5.2 nM. The binding site density for [3H]RX821002 was significantly lower in the neonatal rat lung compared with [3H]rauwolscine. The correlation coefficients of pKi values of adrenergic compounds against [3H]RX821002 versus [3H]rauwolscine were close to unity for each tissue. These data clearly show that the two ligands label the same alpha-2 adrenergic receptor population.(ABSTRACT TRUNCATED AT 250 WORDS)


