Enniatin BCAS# 917-13-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 917-13-5 | SDF | Download SDF |
| PubChem ID | 164754 | Appearance | Powder |
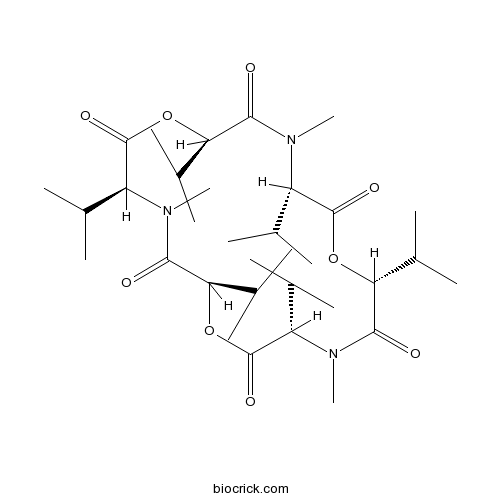
| Formula | C33H57N3O9 | M.Wt | 639.8 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3S,6R,9S,12R,15S,18R)-4,10,16-trimethyl-3,6,9,12,15,18-hexa(propan-2-yl)-1,7,13-trioxa-4,10,16-triazacyclooctadecane-2,5,8,11,14,17-hexone | ||
| SMILES | CC(C)C1C(=O)OC(C(=O)N(C(C(=O)OC(C(=O)N(C(C(=O)OC(C(=O)N1C)C(C)C)C(C)C)C)C(C)C)C(C)C)C)C(C)C | ||
| Standard InChIKey | MIZMDSVSLSIMSC-VYLWARHZSA-N | ||
| Standard InChI | InChI=1S/C33H57N3O9/c1-16(2)22-31(40)43-26(20(9)10)29(38)35(14)24(18(5)6)33(42)45-27(21(11)12)30(39)36(15)23(17(3)4)32(41)44-25(19(7)8)28(37)34(22)13/h16-27H,1-15H3/t22-,23-,24-,25+,26+,27+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Enniatin B can decrease Monocytes' endocytosis ability and an increase of CD71. 2. Enniatin B has anticancer benefits, especially for the treatment of cervical cancer. 3. Enniatin B has extensive hepatic metabolism, that reduces in vivo potential . 4. Enniatin B has antiangiogenic properties, indicated by a strong inhibition of human endothelial cell migration and tube formation. |
| Targets | IL Receptor | P450 (e.g. CYP17) |

Enniatin B Dilution Calculator

Enniatin B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.563 mL | 7.8149 mL | 15.6299 mL | 31.2598 mL | 39.0747 mL |
| 5 mM | 0.3126 mL | 1.563 mL | 3.126 mL | 6.252 mL | 7.8149 mL |
| 10 mM | 0.1563 mL | 0.7815 mL | 1.563 mL | 3.126 mL | 3.9075 mL |
| 50 mM | 0.0313 mL | 0.1563 mL | 0.3126 mL | 0.6252 mL | 0.7815 mL |
| 100 mM | 0.0156 mL | 0.0781 mL | 0.1563 mL | 0.3126 mL | 0.3907 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 5,7,4'-Trihydroxy-8-methylflavanone
Catalog No.:BCN2844
CAS No.:916917-28-7
- TC-I 15
Catalog No.:BCC6216
CAS No.:916734-43-5
- Clematiunicinoside E
Catalog No.:BCN7809
CAS No.:916649-92-8
- Clematomandshurica saponin B
Catalog No.:BCN7810
CAS No.:916649-91-7
- Coriatin
Catalog No.:BCN4457
CAS No.:91653-75-7
- Zingiberen newsaponin
Catalog No.:BCN2942
CAS No.:91653-50-8
- Senkyunolide C
Catalog No.:BCC9141
CAS No.:91652-78-7
- Clinodiside A
Catalog No.:BCN1048
CAS No.:916347-31-4
- Acetylvirolin
Catalog No.:BCN7041
CAS No.:916264-22-7
- Gopherenediol
Catalog No.:BCN6582
CAS No.:916236-79-8
- AC 55541
Catalog No.:BCC3951
CAS No.:916170-19-9
- TCS 1102
Catalog No.:BCC4063
CAS No.:916141-36-1
- 2-[(Acetylthio)methyl]-phenylpropionic acid
Catalog No.:BCC8507
CAS No.:91702-98-6
- CYT997 (Lexibulin)
Catalog No.:BCC4601
CAS No.:917111-44-5
- Bromfenac Sodium
Catalog No.:BCC4641
CAS No.:91714-93-1
- MPC 6827 hydrochloride
Catalog No.:BCC8040
CAS No.:917369-31-4
- Letermovir
Catalog No.:BCC1700
CAS No.:917389-32-3
- Cyclo(Ile-Leu)
Catalog No.:BCN2434
CAS No.:91741-17-2
- Platycoside M1
Catalog No.:BCN3238
CAS No.:917482-67-8
- Platycoside M3
Catalog No.:BCN3243
CAS No.:917482-69-0
- PSB 0474
Catalog No.:BCC7459
CAS No.:917567-60-3
- CCMI
Catalog No.:BCC7788
CAS No.:917837-54-8
- MK-2461
Catalog No.:BCC3816
CAS No.:917879-39-1
- MK-6892
Catalog No.:BCC1767
CAS No.:917910-45-3
Effects of beauvericin, enniatin b and moniliformin on human dendritic cells and macrophages: an in vitro study.[Pubmed:23685117]
Toxicon. 2013 Sep;71:1-10.
The aim of this study was to assess the in vitro effects of emerging mycotoxins beauvericin, Enniatin B and moniliformin on human dendritic cells and macrophages. Beauvericin and Enniatin B were cytotoxic on these cells. IC50 were equal to 1.0 muM, 2.9 muM and 2.5 muM beauvericin for immature dendritic cells, mature dendritic cells and macrophages, respectively. IC50 were equal to 1.6 muM, 2.6 muM and 2.5 muM for immature dendritic cells, mature dendritic cells and macrophages exposed to Enniatin B, respectively. Effects on the differentiation process of monocytes into macrophages or into immature dendritic cells as well as effects on dendritic cells maturation have been studied. The differentiation process of monocytes into immature dendritic cells was not disturbed in the presence of beauvericin. Dendritic cells exposed to beauvericin during the maturation process presented a decrease of CCR7 expression and an increase of IL-10 secretion. Monocytes exposed to beauvericin during the differentiation process into macrophages presented a decrease of endocytosis ability. The differentiation process of monocytes into immature dendritic cells was not disturbed in the presence of Enniatin B. Dendritic cells exposed to Enniatin B during the maturation process presented a decrease of expression of the maturation makers CD80, CD86 and CCR7 and an increase of IL-10 secretion. Monocytes exposed to Enniatin B during the differentiation process into macrophages presented a decrease of endocytosis ability and an increase of CD71. CD1a expression and endocytosis capacity were decreased on immature dendritic cells exposed to moniliformin. Monocytes-derived macrophages exposed to moniliformin during the differentiation process presented a decrease of endocytosis ability, and a decrease of CD71 and HLA-DR expression. According to these results, immunological disorders could be observed on human after ingestion of these alimentary toxins.
An investigation of the endocrine disrupting potential of enniatin B using in vitro bioassays.[Pubmed:25625232]
Toxicol Lett. 2015 Mar 4;233(2):84-94.
Evidence that some of the fungal metabolites present in food and feed may act as potential endocrine disruptors is increasing. Enniatin B (ENN B) is among the emerging Fusarium mycotoxins known to contaminate cereals. In this study, the H295R and neonatal porcine Leydig cell (LC) models, and reporter gene assays (RGAs) have been used to investigate the endocrine disrupting activity of ENN B. Aspects of cell viability, cell cycle distribution, hormone production as well as the expression of key steroidogenic genes were assessed using the H295R cell model. Cell viability and hormone production levels were determined in the LC model, while cell viability and steroid hormone nuclear receptor transcriptional activity were measured using the RGAs. ENN B (0.01-100muM) was cytotoxic in the H295R and LC models used; following 48h incubation with 100muM. Flow cytometry analysis showed that ENN B exposure (0.1-25muM) led to an increased proportion of cells in the S phase at higher ENN B doses (>10muM) while cells at G0/G1 phase were reduced. At the receptor level, ENN B (0.00156-15.6muM) did not appear to induce any specific (ant) agonistic responses in reporter gene assays (RGAs), however cell viability was affected at 15.6muM. Measurement of hormone levels in H295R cells revealed that the production of progesterone, testosterone and cortisol in exposed cells were reduced, but the level of estradiol was not significantly affected. There was a general reduction of estradiol and testosterone levels in exposed LC. Only the highest dose (100muM) used had a significant effect, suggesting the observed inhibitory effect is more likely associated with the cytotoxic effect observed at this dose. Gene transcription analysis in H295R cells showed that twelve of the sixteen genes were significantly modulated (p<0.05) by ENN B (10muM) compared to the control. Genes HMGR, StAR, CYP11A, 3betaHSD2 and CYP17 were downregulated, whereas the expression of CYP1A1, NR0B1, MC2R, CYP21, CYP11B1, CYP11B2 and CYP19 were upregulated. The reduction of hormones and modulation of genes at the lower dose (10muM) in the H295R cells suggests that adrenal endocrine toxicity is an important potential hazard.
The naturally born fusariotoxin enniatin B and sorafenib exert synergistic activity against cervical cancer in vitro and in vivo.[Pubmed:25557295]
Biochem Pharmacol. 2015 Feb 1;93(3):318-331.
During the last decades substantial progress has been made in developing systemic cancer therapy. However, tumors are frequently intrinsically resistant against structurally and mechanistically unrelated drugs. Thus, it is of predominant interest to overcome drug resistance and to encourage the research for novel chemotherapeutic approaches. Recently, we have introduced enniatins, naturally occurring cyclohexadepsipeptides produced by filamentous fungi of the genus Fusarium, as potential anticancer drugs. Here, we expend this approach by demonstrating antiangiogenic properties for Enniatin B (Enn B) indicated by a strong inhibition of human endothelial cell migration and tube formation. Moreover, combination of Enn B with the clinically approved multi-kinase inhibitor sorafenib (Sora) displayed profound synergistic in vitro and in vivo anticancer effects against cervical cancer. Subsequent studies showed that this strong synergism is accompanied by a marked increase in mitochondrial injury and apoptosis induction reflected by mitochondrial membrane depolarization, caspase-7 activation, and subsequent cleavage of PARP. Additionally, cells were shown to stop DNA synthesis and accumulate in S and G2/M phase of the cell cycle. The multifaceted characteristics underlying this strong synergism were suggested to be based on interference with the p38 MAPK as well as the ERK signaling pathways. Finally, also in vivo studies revealed that the combination treatment is distinctly superior to single drug treatments against the KB-3-1 cervix carcinoma xenograft model. Taken together, our data confirm the anticancer benefits of the naturally occurring fusariotoxin Enn B and further present Enn B/Sora as a novel combination strategy especially for the treatment of cervical cancer.
In vitro metabolism of the mycotoxin enniatin B in different species and cytochrome p450 enzyme phenotyping by chemical inhibitors.[Pubmed:21622627]
Drug Metab Dispos. 2011 Sep;39(9):1768-76.
Enniatins are cyclic hexapeptidic mycotoxins produced by fungi growing on field grains, especially in wet climates. They show considerable resistance to food and feed processing technologies and might cause intoxication of humans and animals. Enniatins are also under exploration as anticancer drugs. The observed difference of in vitro and in vivo toxicities suggests low absorption or fast elimination of the enniatins after oral uptake. In the study presented here, in vitro metabolism studies of Enniatin B were performed using rat, dog, and human liver microsomes under conditions of linear kinetics to estimate the respective elimination rates. Furthermore, cytochrome P450 reaction phenotyping with chemical inhibitors selective for human enzymes was carried out. Twelve metabolites were separated and characterized by multiple high-performance liquid chromatographic/mass spectrometric analyses as products of oxidation and demethylation reactions. Biotransformation rates and metabolite patterns varied considerably in the three species. The intrinsic clearances determined in assays with rat, dog, and human liver microsomes were 1.16, 8.23, and 1.13 l/(h . kg), respectively. The predicted Enniatin B in vivo blood clearances were 1.57 l/(h . kg) in rats, 1.67 l/(h . kg) in dogs, and 0.63 l/(h . kg) in humans. CYP3A4 was important for Enniatin B metabolism in human microsomes as shown by 80% inhibition and impaired metabolite formation in the presence of troleandomycin. CYP1A2 and CYP2C19 were additionally involved. Preliminary results showed that CYP3A and CYP1A might also be relevant in rats and dogs. The extensive hepatic metabolism could explain the reduced in vivo potential of Enniatin B.


