PSB 0474Potent and selective P2Y6 agonist CAS# 917567-60-3 |
- PSI
Catalog No.:BCC1124
CAS No.:158442-41-2
- Salinosporamide A (NPI-0052, Marizomib)
Catalog No.:BCC2094
CAS No.:437742-34-2
- Aclacinomycin A
Catalog No.:BCC1232
CAS No.:57576-44-0
- E-64
Catalog No.:BCC1222
CAS No.:66701-25-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 917567-60-3 | SDF | Download SDF |
| PubChem ID | 76156008 | Appearance | Powder |
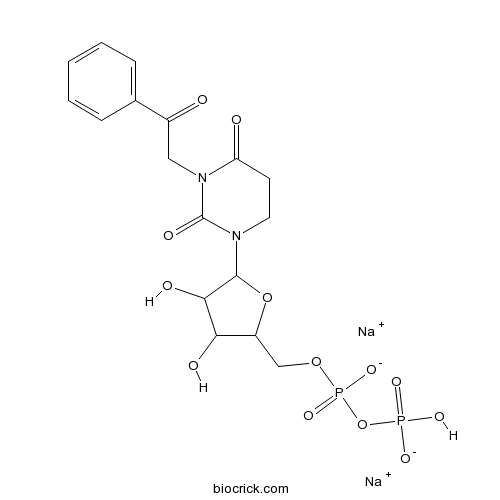
| Formula | C17H18N2Na2O13P2 | M.Wt | 566.26 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 3-Phenacyl UDP disodium salt | ||
| Solubility | Soluble in water (supplied pre-dissolved at a concentration of 10mM) | ||
| Chemical Name | disodium;[[5-(2,4-dioxo-3-phenacyl-1,3-diazinan-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-oxidophosphoryl] hydrogen phosphate | ||
| SMILES | C1CN(C(=O)N(C1=O)CC(=O)C2=CC=CC=C2)C3C(C(C(O3)COP(=O)([O-])OP(=O)(O)[O-])O)O.[Na+].[Na+] | ||
| Standard InChIKey | HHZRPHOJBJBHLG-UHFFFAOYSA-L | ||
| Standard InChI | InChI=1S/C17H22N2O13P2.2Na/c20-11(10-4-2-1-3-5-10)8-19-13(21)6-7-18(17(19)24)16-15(23)14(22)12(31-16)9-30-34(28,29)32-33(25,26)27;;/h1-5,12,14-16,22-23H,6-9H2,(H,28,29)(H2,25,26,27);;/q;2*+1/p-2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective P2Y6 receptor agonist (EC50 values are 70, > 1000 and > 10000 nM for P2Y6, P2Y2 and P2Y4 receptors respectively). Induces contractions of rat isolated intrapulmonary arteries in vitro. |

PSB 0474 Dilution Calculator

PSB 0474 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.766 mL | 8.8299 mL | 17.6597 mL | 35.3195 mL | 44.1493 mL |
| 5 mM | 0.3532 mL | 1.766 mL | 3.5319 mL | 7.0639 mL | 8.8299 mL |
| 10 mM | 0.1766 mL | 0.883 mL | 1.766 mL | 3.5319 mL | 4.4149 mL |
| 50 mM | 0.0353 mL | 0.1766 mL | 0.3532 mL | 0.7064 mL | 0.883 mL |
| 100 mM | 0.0177 mL | 0.0883 mL | 0.1766 mL | 0.3532 mL | 0.4415 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Platycoside M3
Catalog No.:BCN3243
CAS No.:917482-69-0
- Platycoside M1
Catalog No.:BCN3238
CAS No.:917482-67-8
- Cyclo(Ile-Leu)
Catalog No.:BCN2434
CAS No.:91741-17-2
- Letermovir
Catalog No.:BCC1700
CAS No.:917389-32-3
- MPC 6827 hydrochloride
Catalog No.:BCC8040
CAS No.:917369-31-4
- Bromfenac Sodium
Catalog No.:BCC4641
CAS No.:91714-93-1
- CYT997 (Lexibulin)
Catalog No.:BCC4601
CAS No.:917111-44-5
- 2-[(Acetylthio)methyl]-phenylpropionic acid
Catalog No.:BCC8507
CAS No.:91702-98-6
- Enniatin B
Catalog No.:BCN4774
CAS No.:917-13-5
- 5,7,4'-Trihydroxy-8-methylflavanone
Catalog No.:BCN2844
CAS No.:916917-28-7
- TC-I 15
Catalog No.:BCC6216
CAS No.:916734-43-5
- Clematiunicinoside E
Catalog No.:BCN7809
CAS No.:916649-92-8
- CCMI
Catalog No.:BCC7788
CAS No.:917837-54-8
- MK-2461
Catalog No.:BCC3816
CAS No.:917879-39-1
- MK-6892
Catalog No.:BCC1767
CAS No.:917910-45-3
- UCL 2077
Catalog No.:BCC7446
CAS No.:918311-87-2
- Cefdinir
Catalog No.:BCC3747
CAS No.:91832-40-5
- 5,7,4'-Trimethoxyafzelechin
Catalog No.:BCN7933
CAS No.:918428-88-3
- Vemurafenib (PLX4032, RG7204)
Catalog No.:BCC1269
CAS No.:918504-65-1
- BRAF inhibitor
Catalog No.:BCC1436
CAS No.:918505-61-0
- PLX-4720
Catalog No.:BCC1280
CAS No.:918505-84-7
- TH-302
Catalog No.:BCC1998
CAS No.:918633-87-1
- GPi 688
Catalog No.:BCC6091
CAS No.:918902-32-6
- 19-[(beta-D-glucopyranosyl)oxy]-19-oxo-ent-labda-8(17),13-dien-16,15-olide
Catalog No.:BCN1308
CAS No.:919120-78-8
Microglia P2Y(6) receptors mediate nitric oxide release and astrocyte apoptosis.[Pubmed:25178395]
J Neuroinflammation. 2014 Sep 3;11:141.
BACKGROUND: During cerebral inflammation uracil nucleotides leak to the extracellular medium and activate glial pyrimidine receptors contributing to the development of a reactive phenotype. Chronically activated microglia acquire an anti-inflammatory phenotype that favors neuronal differentiation, but the impact of these microglia on astrogliosis is unknown. We investigated the contribution of pyrimidine receptors to microglia-astrocyte signaling in a chronic model of inflammation and its impact on astrogliosis. METHODS: Co-cultures of astrocytes and microglia were chronically treated with lipopolysaccharide (LPS) and incubated with uracil nucleotides for 48 h. The effect of nucleotides was evaluated in methyl-[3H]-thymidine incorporation. Western blot and immunofluorescence was performed to detect the expression of P2Y6 receptors and the inducible form of nitric oxide synthase (iNOS). Nitric oxide (NO) release was quantified through Griess reaction. Cell death was also investigated by the LDH assay and by the TUNEL assay or Hoechst 33258 staining. RESULTS: UTP, UDP (0.001 to 1 mM) or PSB 0474 (0.01 to 10 muM) inhibited cell proliferation up to 43 +/- 2% (n = 10, P <0.05), an effect prevented by the selective P2Y6 receptor antagonist MRS 2578 (1 muM). UTP was rapidly metabolized into UDP, which had a longer half-life. The inhibitory effect of UDP (1 mM) was abolished by phospholipase C (PLC), protein kinase C (PKC) and nitric oxide synthase (NOS) inhibitors. Both UDP (1 mM) and PSB 0474 (10 muM) increased NO release up to 199 +/- 20% (n = 4, P <0.05), an effect dependent on P2Y6 receptors-PLC-PKC pathway activation, indicating that this pathway mediates NO release. Western blot and immunocytochemistry analysis indicated that P2Y6 receptors were expressed in the cultures being mainly localized in microglia. Moreover, the expression of iNOS was mainly observed in microglia and was upregulated by UDP (1 mM) or PSB 0474 (10 muM). UDP-mediated NO release induced apoptosis in astrocytes, but not in microglia. CONCLUSIONS: In LPS treated co-cultures of astrocytes and microglia, UTP is rapidly converted into UDP, which activates P2Y6 receptors inducing the release of NO by microglia that causes astrocyte apoptosis, thus controlling their rate of proliferation and preventing an excessive astrogliosis.
Identification of contractile P2Y1, P2Y6, and P2Y12 receptors in rat intrapulmonary artery using selective ligands.[Pubmed:22991416]
J Pharmacol Exp Ther. 2012 Dec;343(3):755-62.
ATP and UDP constrict rat intrapulmonary arteries, but which receptors mediate these actions is unclear. Here, we used selective agonists and antagonists, along with measurements of P2Y receptor expression, to characterize the receptor subtypes involved. Isometric tension was recorded from endothelium-denuded rat intrapulmonary artery rings (i.d. 200-500 mum) mounted on a wire myograph. Expression of P2Y receptor subtype expression was determined by using reverse transcription-polymerase chain reaction with receptor-specific oligonucleotide primers. The selective P2Y(1) agonist (N)-methanocarba-2-methylthioadenosine-5'-O-diphosphate (MRS2365) induced small, concentration-dependent contractions that were inhibited by the P2Y(1) antagonist N(6)-methyl-2'-deoxyadenosine-3',5'-bisphosphate (MRS2179). Contractions evoked by ATP were unaffected by MRS2179, but inhibited by approximately one-third by the P2Y(12) antagonist N(6)-(2-methylthiomethyl)-2-(3,3,3-trifluoropropylthio)dichloro-methylene ATP (AR-C69931MX). Combined blockade of P2X1 and P2Y(12) receptors virtually abolished the response to ATP. ADP also evoked contractions that were abolished by AR-C69931MX. The selective P2Y(6) receptor agonist 3-(2-oxo-2-phenylethyl)-UDP (PSB 0474) evoked concentration-dependent contractions and was approximately three times more potent than UDP, but the P2Y(14) agonist UDP-glucose had no effect. Contractions evoked by UDP were inhibited by the P2Y(6) receptor antagonist N,N'-1,4-butanediylbis-N'-(3-isothiocyanatophenyl)thiourea (MRS2578), but not the cysteinyl leukotriene 1 (CysL(1)) antagonist 3-(3-(2-(7-chloro-2-quinolinyl)ethenyl)phenyl)((3-dimethylamino-3-oxopropyl)thio) methyl)thiopropanoic acid (MK571). Higher concentrations of MRS2578 inhibited contractions to KCl, so they were not studied further. mRNA for P2Y(1), P2Y(6), and P2Y(12) receptors was identified. Our working model is that P2Y(12) and P2X1 receptors are present in rat intrapulmonary arteries and together mediate ATP-induced vasoconstriction. Contractile P2Y(6), but not P2Y(14) or CysLT(1), receptors are also present and are a major site through which UDP evokes constriction.
Pharmacological characterization of uracil nucleotide-preferring P2Y receptors modulating intestinal motility: a study on mouse ileum.[Pubmed:22102167]
Purinergic Signal. 2012 Jun;8(2):275-85.
We investigated the possible modulation of the intestinal contractility by uracil nucleotides (UTP and UDP), using as model the murine small intestine. Contractile activity of a mouse ileum longitudinal muscle was examined in vitro as changes in isometric tension. Transcripts encoding for uracil-sensitive receptors was investigated by RT-PCR. UDP induced muscular contractions, sensitive to PPADS, suramin, or MRS 2578, P2Y(6) receptor antagonist, and mimicked by PSB 0474, P2Y(6)-receptor agonist. UTP induced biphasic effects characterized by an early inhibition of the spontaneous contractile activity followed by muscular contraction. UTP excitatory effects were antagonized by PPADS, suramin, but not by MRS 2578, whilst the inhibitory effects were antagonized by PPADS but not by suramin or MRS 2578. UTPgammaS, P2Y(2)/(4) receptor agonist but not 2-thio-UTP, P2Y(2) receptor agonist, mimicked UTP effects. The inhibitory effects induced by UTP was abolished by ATP desensitization and increased by extracellular acidification. UDP or UTP responses were insensitive to TTX, atropine, or L-NAME antagonized by U-73122, inhibitor of phospholipase C (PLC) and preserved in the presence of nifedipine or low Ca(2+) solution. Transcripts encoding the uracil nucleotide-preferring receptors were expressed in mouse ileum. Functional postjunctional uracil-sensitive receptors are present in the longitudinal muscle of the mouse ileum. Activation of P2Y(6) receptors induces muscular contraction, whilst activation of P2Y(4) receptors leads to inhibition of the contractile activity. Indeed, the presence of atypical UTP-sensitive receptors leading to muscular contraction is suggested. All uracil-sensitive receptors are linked to the PLC pathway.
Role of ecto-NTPDases on UDP-sensitive P2Y(6) receptor activation during osteogenic differentiation of primary bone marrow stromal cells from postmenopausal women.[Pubmed:21898410]
J Cell Physiol. 2012 Jun;227(6):2694-709.
This study aimed at investigating the expression and function of uracil nucleotide-sensitive receptors (P2Y(2), P2Y(4), and P2Y(6)) on osteogenic differentiation of human bone marrow stromal cells (BMSCs) in culture. Bone marrow specimens were obtained from postmenopausal female patients (68 +/- 5 years old, n = 18) undergoing total hip arthroplasty. UTP and UDP (100 microM) facilitated osteogenic differentiation of the cells measured as increases in alkaline phosphatase (ALP) activity, without affecting cell proliferation. Uracil nucleotides concentration-dependently increased [Ca(2+)](i) in BMSCs; their effects became less evident with time (7 > 21 days) of the cells in culture. Selective activation of P2Y(6) receptors with the stable UDP analog, PSB 0474, mimicked the effects of both UTP and UDP, whereas UTPgammaS was devoid of effect. Selective blockade of P2Y(6) receptors with MRS 2578 prevented [Ca(2+)](i) rises and osteogenic differentiation caused by UDP at all culture time points. BMSCs are immunoreactive against P2Y(2), P2Y(4), and P2Y(6) receptors. While the expression of P2Y(6) receptors remained fairly constant (7 approximately 21 days), P2Y(2) and P2Y(4) became evident only in less proliferative and more differentiated cultures (7 < 21 days). The rate of extracellular UTP and UDP inactivation was higher in less proliferative and more differentiated cell populations. Immunoreactivity against NTPDase1, -2, and -3 rises as cells differentiate (7 < 21 days). Data show that uracil nucleotides are important regulators of osteogenic cells differentiation predominantly through the activation of UDP-sensitive P2Y(6) receptors coupled to increases in [Ca(2+)](i) . Endogenous actions of uracil nucleotides may be balanced through specific NTPDases determining whether osteoblast progenitors are driven into proliferation or differentiation.
Synthesis and structure-activity relationships of uracil nucleotide derivatives and analogues as agonists at human P2Y2, P2Y4, and P2Y6 receptors.[Pubmed:17125260]
J Med Chem. 2006 Nov 30;49(24):7076-87.
A series of UTP, UDP, and UMP derivatives and analogues were synthesized and evaluated at the human pyrimidinergic P2Y receptor subtypes P2Y2, P2Y4, and P2Y6 stably expressed in 1321N1 astrocytoma cells. Substituents at N3 of UTP were poorly tolerated by P2Y2 and P2Y4 receptors. In contrast, a large phenacyl substituent at N3 of UDP was well tolerated by the P2Y6 receptor, yielding a potent and selective P2Y6 receptor agonist (3-phenacyl-UDP, EC50=70 nM, >500-fold selective). The most potent and selective P2Y2 receptor agonist of the present series was 2-thio-UTP (EC50=50 nM, >or=30-fold selective vs P2Y4 and P2Y6). All modifications at the uracil base of UTP led to a decrease in potency at the P2Y4 receptor. A beta,gamma-dichloromethylene modification in the triphosphate chain of 5-bromo-UTP was tolerated by all three receptor subtypes, thus opening up a new strategy to obtain ectonucleotide diphosphohydrolase- and phosphatase-resistant P2Y2, P2Y4, and P2Y6 receptor agonists.


