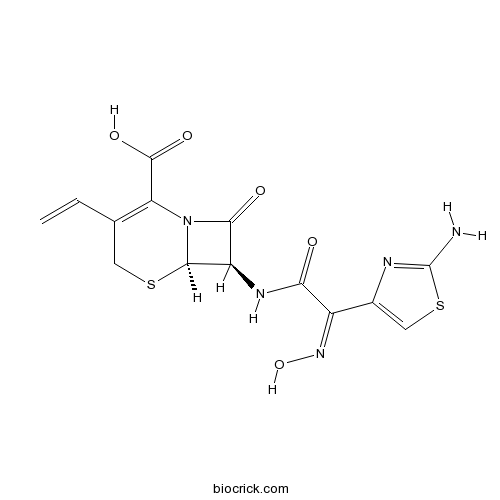
CefdinirCAS# 91832-40-5 |
- Alvimopan monohydrate
Catalog No.:BCC1349
CAS No.:1383577-62-5
- Alvimopan
Catalog No.:BCC1347
CAS No.:156053-89-3
- Alvimopan dihydrate
Catalog No.:BCC1348
CAS No.:170098-38-1
- JDTic
Catalog No.:BCC1670
CAS No.:361444-66-8
- ADL5859 HCl
Catalog No.:BCC1265
CAS No.:850173-95-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 91832-40-5 | SDF | Download SDF |
| PubChem ID | 6915944 | Appearance | Powder |
| Formula | C14H13N5O5S2 | M.Wt | 395.41 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 33.33 mg/mL (84.29 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | (6R,7R)-7-[[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-hydroxyiminoacetyl]amino]-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid | ||
| SMILES | Nc1scc(n1)C(=NO)C(=O)N[C@H]2[C@H]3SCC(=C(N3C2=O)C(O)=O)C=C | ||
| Standard InChIKey | RTXOFQZKPXMALH-GHXIOONMSA-N | ||
| Standard InChI | InChI=1S/C14H13N5O5S2/c1-2-5-3-25-12-8(11(21)19(12)9(5)13(22)23)17-10(20)7(18-24)6-4-26-14(15)16-6/h2,4,8,12,24H,1,3H2,(H2,15,16)(H,17,20)(H,22,23)/b18-7-/t8-,12-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Cefdinir Dilution Calculator

Cefdinir Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.529 mL | 12.6451 mL | 25.2902 mL | 50.5804 mL | 63.2255 mL |
| 5 mM | 0.5058 mL | 2.529 mL | 5.058 mL | 10.1161 mL | 12.6451 mL |
| 10 mM | 0.2529 mL | 1.2645 mL | 2.529 mL | 5.058 mL | 6.3226 mL |
| 50 mM | 0.0506 mL | 0.2529 mL | 0.5058 mL | 1.0116 mL | 1.2645 mL |
| 100 mM | 0.0253 mL | 0.1265 mL | 0.2529 mL | 0.5058 mL | 0.6323 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Cefdinir is a Cephalosporin antibiotic that is structurally similar to Cefixime.
- UCL 2077
Catalog No.:BCC7446
CAS No.:918311-87-2
- MK-6892
Catalog No.:BCC1767
CAS No.:917910-45-3
- MK-2461
Catalog No.:BCC3816
CAS No.:917879-39-1
- CCMI
Catalog No.:BCC7788
CAS No.:917837-54-8
- PSB 0474
Catalog No.:BCC7459
CAS No.:917567-60-3
- Platycoside M3
Catalog No.:BCN3243
CAS No.:917482-69-0
- Platycoside M1
Catalog No.:BCN3238
CAS No.:917482-67-8
- Cyclo(Ile-Leu)
Catalog No.:BCN2434
CAS No.:91741-17-2
- Letermovir
Catalog No.:BCC1700
CAS No.:917389-32-3
- MPC 6827 hydrochloride
Catalog No.:BCC8040
CAS No.:917369-31-4
- Bromfenac Sodium
Catalog No.:BCC4641
CAS No.:91714-93-1
- CYT997 (Lexibulin)
Catalog No.:BCC4601
CAS No.:917111-44-5
- 5,7,4'-Trimethoxyafzelechin
Catalog No.:BCN7933
CAS No.:918428-88-3
- Vemurafenib (PLX4032, RG7204)
Catalog No.:BCC1269
CAS No.:918504-65-1
- BRAF inhibitor
Catalog No.:BCC1436
CAS No.:918505-61-0
- PLX-4720
Catalog No.:BCC1280
CAS No.:918505-84-7
- TH-302
Catalog No.:BCC1998
CAS No.:918633-87-1
- GPi 688
Catalog No.:BCC6091
CAS No.:918902-32-6
- 19-[(beta-D-glucopyranosyl)oxy]-19-oxo-ent-labda-8(17),13-dien-16,15-olide
Catalog No.:BCN1308
CAS No.:919120-78-8
- 1-Methoxyindole-3-carboxylic acid
Catalog No.:BCN3946
CAS No.:91913-76-7
- Atrial natriuretic factor (1-28) (human, porcine)
Catalog No.:BCC5839
CAS No.:91917-63-4
- Ro 15-4513
Catalog No.:BCC7230
CAS No.:91917-65-6
- Rubrisandrin A
Catalog No.:BCN3248
CAS No.:919289-30-8
- AZD1283
Catalog No.:BCC5370
CAS No.:919351-41-0
Cefdinir Solid Dispersion Composed of Hydrophilic Polymers with Enhanced Solubility, Dissolution, and Bioavailability in Rats.[Pubmed:28208830]
Molecules. 2017 Feb 13;22(2). pii: molecules22020280.
The aim of this work was to develop Cefdinir solid dispersions (CSDs) prepared using hydrophilic polymers with enhanced dissolution/solubility and in vivo oral bioavailability. CSDs were prepared with hydrophilic polymers such as hydroxypropyl-methylcellulose (HPMC; CSD1), carboxymethylcellulose-Na (CMC-Na; CSD2), polyvinyl pyrrolidone K30 (PVP K30; CSD3) at the weight ratio of 1:1 (drug:polymer) using a spray-drying method. The prepared CSDs were characterized by aqueous solubility, differential scanning calorimetry (DSC), powder X-ray diffraction (p-XRD), scanning electron microscopy (SEM), aqueous viscosity, and dissolution test in various media. The oral bioavailability of CSDs was also evaluated in rats and compared with Cefdinir powder suspension. The Cefdinir in CSDs was amorphous form, as confirmed in the DSC and p-XRD measurements. The developed CSDs commonly resulted in about 9.0-fold higher solubility of Cefdinir and a significantly improved dissolution profile in water and at pH 1.2, compared with Cefdinir crystalline powder. Importantly, the in vivo oral absorption (represented as AUCinf) was markedly increased by 4.30-, 6.77- and 3.01-fold for CSD1, CSD2, and CSD3, respectively, compared with Cefdinir suspension in rats. The CSD2 prepared with CMC-Na would provide a promising vehicle to enhance dissolution and bioavailability of Cefdinir in vivo.
[Construction of the quantitative structure retention relationship of cefdinir related substances].[Pubmed:26757554]
Yao Xue Xue Bao. 2015 Sep;50(9):1161-6.
The molecular descriptors of impurities with known structure in Cefdinir were calculated, selected and associated with the chromatographic retention behavior to establish a model. This quantitative structure retention relationships (QSRR) model for the related substances of Cefdinir was established under specific chromatographic condition and verified by other impurities. 12 molecular descriptors were used to establish the QSRR model, F_AFRBWF, Blbn_J, SsCH3, SssCH2, SsNH2, SssNH, SssS, SHdCH2, EEM_AFc, EEM_AFpl, EEM_XFpl and Pi_MaxQ. The relativity between true values and predictions in QSRR of Cefdinir is R2 = 0.9836 (n = 18), DeltaRRT is no more than 0.154, as 10.17% in RRT. The results indicate that the QSRR model for the related substances of Cefdinir can be used to evaluate the analysis methods for related substances and predict the chromatographic behavior of new impurities, which will provide a new way for the evaluation of the effectiveness for drug quality control.
Combining the FtsZ-Targeting Prodrug TXA709 and the Cephalosporin Cefdinir Confers Synergy and Reduces the Frequency of Resistance in Methicillin-Resistant Staphylococcus aureus.[Pubmed:27161635]
Antimicrob Agents Chemother. 2016 Jun 20;60(7):4290-6.
Combination therapy of bacterial infections with synergistic drug partners offers distinct advantages over monotherapy. Among these advantages are (i) a reduction of the drug dose required for efficacy, (ii) a reduced potential for drug-induced toxicity, and (iii) a reduced potential for the emergence of resistance. Here, we describe the synergistic actions of the third-generation oral cephalosporin Cefdinir and TXA709, a new, FtsZ-targeting prodrug that we have developed with improved pharmacokinetics and enhanced in vivo efficacy against methicillin-resistant Staphylococcus aureus (MRSA) relative to earlier agents. We show that the active product of TXA709 (TXA707) acts synergistically with Cefdinir in vitro against clinical isolates of MRSA, vancomycin-intermediate S. aureus (VISA), vancomycin-resistant S. aureus (VRSA), and linezolid-resistant S. aureus (LRSA). In addition, relative to TXA707 alone, the combination of TXA707 and Cefdinir significantly reduces or eliminates the detectable emergence of resistance. We also demonstrate synergy in vivo with oral administration of the prodrug TXA709 and Cefdinir in mouse models of both systemic and tissue (thigh) infections with MRSA. This synergy reduces the dose of TXA709 required for efficacy 3-fold. Viewed as a whole, our results highlight the potential of TXA709 and Cefdinir as a promising combination for the treatment of drug-resistant staphylococcal infections.
Application and optimization of organic-inorganic hybrid monolithic capillary electrochromatography for in vivo cefdinir determination with microdialysis.[Pubmed:26549514]
J Sep Sci. 2016 Jan;39(2):440-9.
In this study, an organic-inorganic hybrid monolithic capillary column was applied and optimized for the determination of Cefdinir in plasma, and the electro-osmotic flow that usually hinders migration in reverse polarity became a driving force. The Sample used for pharmacokinetic research was collected by microdialysis using phosphate buffer (pH 7.4) as perfusate, and a volume of 60 muL fluid was mixed with 140 muL of acetonitrile. By using a silica-allyldimethyldodecylammonium monolithic column (100 mum inner diameter, 21 cm effective length and 31.2 cm total length), and a mobile phase consisting of phosphate and acetonitrile (pH 4.5, 50:50, v/v), at a voltage of 20 kV, the analytes were successfully separated with the background within 2.5 min. The detection wavelength was 214 nm. The calibration curve showed a good linearity (r(2) = 0.9994) over the concentration range of 0.2-50 mug/mL. The proposed method showed good specificity, linearity, sensitivity, precision and recovery, and the introduction of field amplified sample stacking helped to improve the low recovery caused by microdialysis. This method was successfully applied to quantify Cefdinir in rat plasma to support a pre-clinical pharmacokinetic trial.


