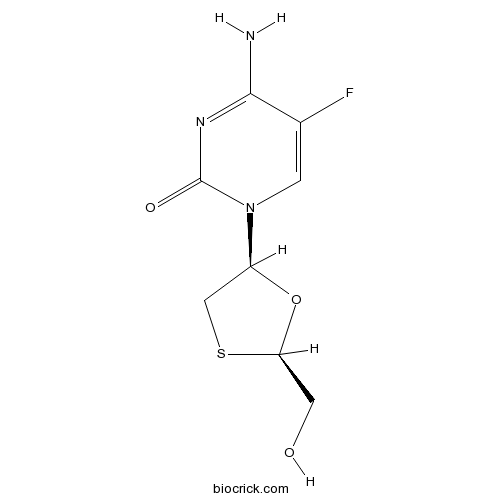
EmtricitabineCAS# 143491-57-0 |
- Tenofovir
Catalog No.:BCC2500
CAS No.:147127-20-6
- Tenofovir Disoproxil Fumarate
Catalog No.:BCC1108
CAS No.:202138-50-9
- Entecavir Hydrate
Catalog No.:BCC1109
CAS No.:209216-23-9
- Lersivirine
Catalog No.:BCC1698
CAS No.:473921-12-9
- Didanosine
Catalog No.:BCC3763
CAS No.:69655-05-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 143491-57-0 | SDF | Download SDF |
| PubChem ID | 60877 | Appearance | Powder |
| Formula | C8H10FN3O3S | M.Wt | 247.25 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BW1592 | ||
| Solubility | DMSO : 10.8 mg/mL (43.68 mM; Need ultrasonic and warming) | ||
| Chemical Name | 4-amino-5-fluoro-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2-one | ||
| SMILES | C1C(OC(S1)CO)N2C=C(C(=NC2=O)N)F | ||
| Standard InChIKey | XQSPYNMVSIKCOC-NTSWFWBYSA-N | ||
| Standard InChI | InChI=1S/C8H10FN3O3S/c9-4-1-12(8(14)11-7(4)10)5-3-16-6(2-13)15-5/h1,5-6,13H,2-3H2,(H2,10,11,14)/t5-,6+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Emtricitabine is an inhibitor of the nucleoside reverse transcriptase (NRTI) and human immunodeficiency virus type 1 (HIV-1); inhibits NRTI with an EC50 of 0.01 µM in PBMC cell.In Vitro:Emtricitabine has in vitro activity against both laboratory strains of HIV-1 and HIV-2 and clinical isolates of HIV-1. The 50% effective concentration (EC50) ranges from 0.002 to 1.5 µ mol/L, depending on the viral isolate and cell line used. Emtricitabine demonstrates in vitro synergy with zidovudine and stavudine and additive in vitro activity when combines with zalcitabine or didanosine[1].In Vivo:Reproductive and developmental toxicology studies are conducted with emtricitabine. Oral doses up to 1000 mg/kg/day provided daily area under the curve (AUC0→24) exposure to pregnant animals approximately 60- (mice) to 120-fold (rabbits) higher than that in humans at the recommended dose of 200 mg given once per day. In a mouse fertility study, emtricitabine had no effect on fertility, sperm count, or early embryonic development. There is no increased incidence of malformations in mouse and rabbit embryofetal toxicology studies. The development and fertility of F1 progeny are unaffected by emtricitabine in a mouse pre- and post-natal study. These data demonstrate a favorable pre-clinical reproductive safety profile for emtricitabine[2]. References: | |||||

Emtricitabine Dilution Calculator

Emtricitabine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0445 mL | 20.2224 mL | 40.4449 mL | 80.8898 mL | 101.1122 mL |
| 5 mM | 0.8089 mL | 4.0445 mL | 8.089 mL | 16.178 mL | 20.2224 mL |
| 10 mM | 0.4044 mL | 2.0222 mL | 4.0445 mL | 8.089 mL | 10.1112 mL |
| 50 mM | 0.0809 mL | 0.4044 mL | 0.8089 mL | 1.6178 mL | 2.0222 mL |
| 100 mM | 0.0404 mL | 0.2022 mL | 0.4044 mL | 0.8089 mL | 1.0111 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Emtricitabine is a nucleoside analogue which inhibits the reverse nucleoside transcriptase enzyme.
- LOE 908 hydrochloride
Catalog No.:BCC7327
CAS No.:143482-60-4
- 5,8-Dihydroxypsoralen
Catalog No.:BCC8104
CAS No.:14348-23-3
- Cnidilin
Catalog No.:BCN2731
CAS No.:14348-22-2
- Poriol
Catalog No.:BCN6816
CAS No.:14348-16-4
- (Arg)9 peptide
Catalog No.:BCC5336
CAS No.:143413-47-2
- SAR131675
Catalog No.:BCC5097
CAS No.:1433953-83-3
- Naratriptan
Catalog No.:BCC5053
CAS No.:143388-64-1
- Bryostatin 3
Catalog No.:BCC5620
CAS No.:143370-84-7
- SMIP004
Catalog No.:BCC1955
CAS No.:143360-00-3
- RS 56812 hydrochloride
Catalog No.:BCC6877
CAS No.:143339-12-2
- UNC 2400
Catalog No.:BCC5625
CAS No.:1433200-49-7
- Ac-YVAD-CHO
Catalog No.:BCC4021
CAS No.:143313-51-3
- Dammarenediol II
Catalog No.:BCN6240
CAS No.:14351-29-2
- 3-Acetoxy-24-hydroxydammara-20,25-diene
Catalog No.:BCN1569
CAS No.:143519-04-4
- (4S,5R)-3-(tert-Butoxycarbonyl)-2,2-dimethyl-4-phenyloxazolidine-5-carboxylic acid
Catalog No.:BCN8364
CAS No.:143527-70-2
- Shancigusin I
Catalog No.:BCN8272
CAS No.:1435488-35-9
- XMD17-109
Catalog No.:BCC2061
CAS No.:1435488-37-1
- L 012 sodium salt
Catalog No.:BCC6362
CAS No.:143556-24-5
- Rocuronium
Catalog No.:BCC1906
CAS No.:143558-00-3
- Virgatic acid
Catalog No.:BCN6744
CAS No.:14356-51-5
- Diprenorphine
Catalog No.:BCC5954
CAS No.:14357-78-9
- H-DL-Asp(OMe)-OMe.HCl
Catalog No.:BCC2901
CAS No.:14358-33-9
- A 419259 trihydrochloride
Catalog No.:BCC4308
CAS No.:1435934-25-0
- Cyclo(Leu-Leu)
Catalog No.:BCN2433
CAS No.:1436-27-7
Brief Report: Randomized, Double-Blind Comparison of Tenofovir Alafenamide (TAF) vs Tenofovir Disoproxil Fumarate (TDF), Each Coformulated With Elvitegravir, Cobicistat, and Emtricitabine (E/C/F) for Initial HIV-1 Treatment: Week 144 Results.[Pubmed:28282300]
J Acquir Immune Defic Syndr. 2017 Jun 1;75(2):211-218.
In 2 double-blind phase 3 trials, 1733 antiretroviral-naive adults were randomized to tenofovir alafenamide (TAF) or tenofovir disoproxil fumarate (TDF), each coformulated with elvitegravir/cobicistat/Emtricitabine (E/C/F). At 144 weeks, TAF was superior to TDF in virologic efficacy, with 84.2% vs 80.0% having HIV-1 RNA <50 copies/mL (difference 4.2%; 95% confidence interval: 0.6% to 7.8%). TAF had less impact than TDF on bone mineral density and renal biomarkers. No participants on TAF had renal-related discontinuations vs 12 on TDF (P < 0.001), with no cases of proximal tubulopathy for TAF vs 4 for TDF. There were greater increases in lipids with TAF vs TDF, with no difference in the total cholesterol to high-density lipoprotein ratio. For initial HIV therapy, E/C/F/TAF is superior to E/C/F/TDF in efficacy and bone and renal safety.
Dolutegravir with tenofovir disoproxil fumarate-emtricitabine as HIV postexposure prophylaxis in gay and bisexual men.[Pubmed:28301425]
AIDS. 2017 Jun 1;31(9):1291-1295.
OBJECTIVES: Completion rates for HIV postexposure prophylaxis (PEP) are often low. We investigated the adherence and safety of dolutegravir (DTG; 50 mg daily) with tenofovir disoproxil fumarate-Emtricitabine (TDF-FTC; 300/200 mg, respectively) as three-drug PEP in gay and bisexual men. DESIGN: Open-label, single-arm study at three sexual health clinics and two emergency departments in Australia. METHODS: In total, 100 HIV-uninfected gay and bisexual men requiring PEP received DTG and TDF-FTC for 28 days. The primary end point was PEP failure (premature PEP cessation or primary HIV infection through week 12). Additional end points were adherence by self-report (n = 98) and pill count (n = 55), safety, and plasma drug levels at day 28. RESULTS: PEP completion was 90% (95% confidence interval 84-96%). Failures (occurring at a median 9 days, interquartile range 3-16) comprised loss to follow-up (9%) and adverse event resulting in study drug discontinuation (headache, 1%). No participant was found to acquire HIV through week 12. Adherence to PEP was 98% by self-report and in the 55 participants with corresponding pill count data. The most common clinical adverse events were fatigue (26%), nausea (25%), diarrhoea (21%), and headache (10%). There were only four grade 3-4 subjective adverse events. The most common laboratory adverse event was raised alanine aminotransferase (22%), but there was no case of clinical hepatitis. At day 28, the mean estimated glomerular filtration rate decrease was 14 ml/min/1.73m (SD 17, P = 0.001); an estimated glomerular filtration rate of less than 60 ml/min/1.73m occurred in 3%. CONCLUSIONS: DTG with TDF-FTC is a well tolerated option for once-daily PEP.
Efficacy and safety of emtricitabine/tenofovir alafenamide (FTC/TAF) vs. emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) as a backbone for treatment of HIV-1 infection in virologically suppressed adults: subgroup analysis by third agent of a randomized, double-blind, active-controlled phase 3 trial.[Pubmed:28303753]
HIV Clin Trials. 2017 May;18(3):135-140.
BACKGROUND: FTC/TAF was shown to be noninferior to FTC/TDF with advantages in markers of renal and bone safety. OBJECTIVE: To evaluate the efficacy and safety of switching to FTC/TAF from FTC/TDF by third agent (boosted protease inhibitor [PI] vs. unboosted third agent). METHODS: We conducted a 48-week subgroup analysis based on third agent from a randomized, double blind study in virologically suppressed adults on a FTC/TDF-containing regimen who switched to FTC/TAF vs. continued FTC/TDF while remaining on the same third agent. RESULTS: We randomized (1:1) 663 participants to either switch to FTC/TAF (N = 333) or continue FTC/TDF (N = 330), each with baseline third agent stratifying by class of third agent in the prior treatment regimen (boosted PI 46%, unboosted third agent 54%). At week 48, significant differences in renal biomarkers and bone mineral density were observed favoring FTC/TAF over FTC/TDF (p < 0.05 for all), with similar improvements in the FTC/TAF arm in those who received boosted PI vs. unboosted third agents. At week 48, virologic success rates were similar between treatment groups for those who received a boosted PI (FTC/TAF 92%, FTC/TDF 93%) and for those who received an unboosted third agent (97% vs. 93%). CONCLUSIONS: In virologically suppressed patients switching to FTC/TAF from FTC/TDF, high rates of virologic suppression were maintained, while renal and bone safety parameters improved, regardless of whether participants were receiving a boosted PI or an unboosted third agent. FTC/TAF offers safety advantages over FTC/TDF and can be an important option as an NRTI backbone given with a variety of third agents.


