(Arg)9 peptideCell penetrating inhibitor CAS# 143413-47-2 |
- U0126-EtOH
Catalog No.:BCC1066
CAS No.:1173097-76-1
- PD98059
Catalog No.:BCC1098
CAS No.:167869-21-8
- PD184352 (CI-1040)
Catalog No.:BCC1112
CAS No.:212631-79-3
- SL-327
Catalog No.:BCC1123
CAS No.:305350-87-2
- MEK162 (ARRY-162, ARRY-438162)
Catalog No.:BCC1148
CAS No.:606143-89-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 143413-47-2 | SDF | Download SDF |
| PubChem ID | 25077438 | Appearance | Powder |
| Formula | C54H110N36O10 | M.Wt | 1423.69 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Nona-L-arginine; Peptide R9 | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
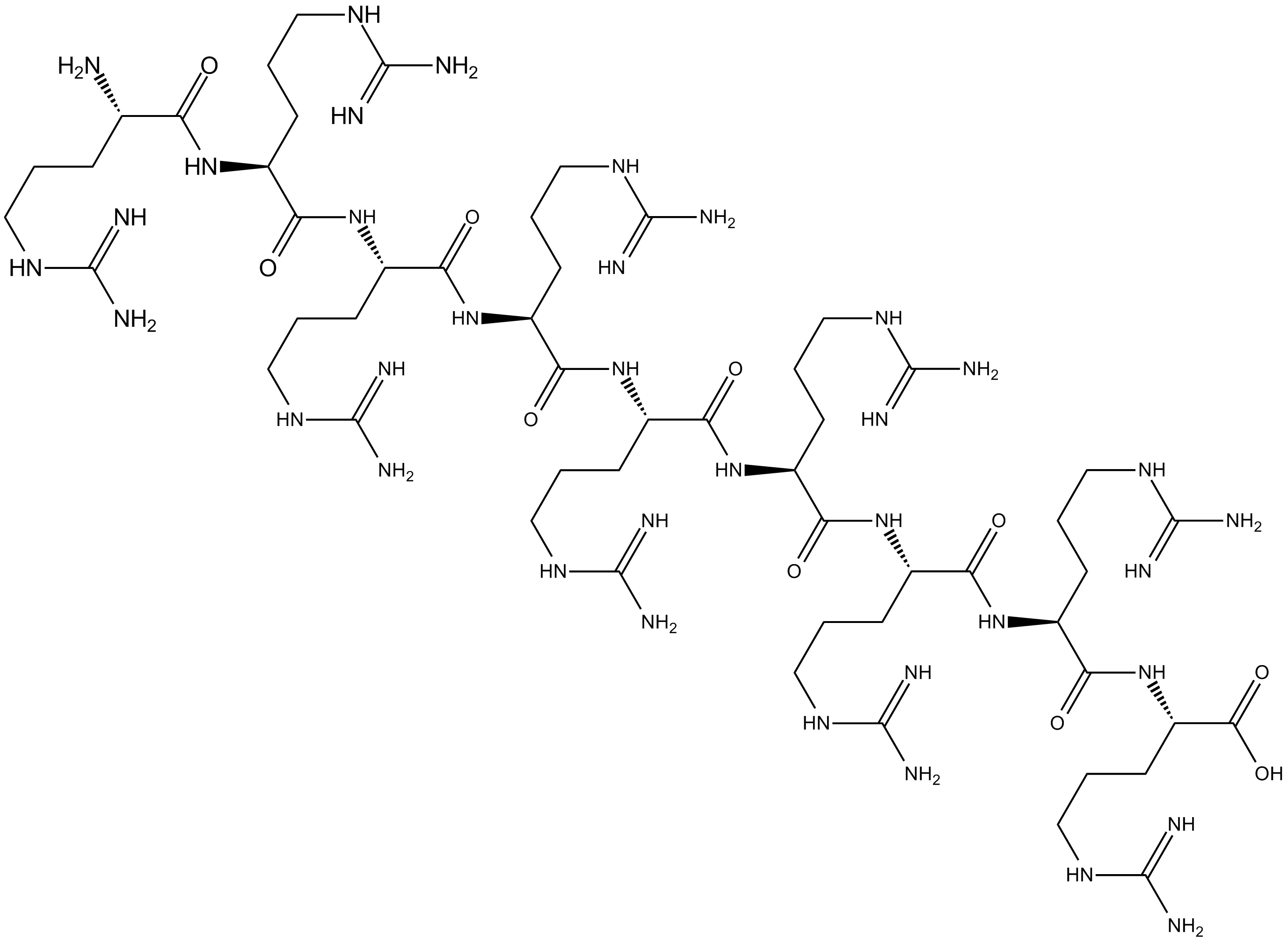
| Chemical Name | (2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-amino-5-(diaminomethylideneamino)pentanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-5-(diaminomethylideneamino)pentanoic acid | ||
| SMILES | C(CC(C(=O)NC(CCCN=C(N)N)C(=O)NC(CCCN=C(N)N)C(=O)NC(CCCN=C(N)N)C(=O)NC(CCCN=C(N)N)C(=O)NC(CCCN=C(N)N)C(=O)NC(CCCN=C(N)N)C(=O)NC(CCCN=C(N)N)C(=O)NC(CCCN=C(N)N)C(=O)O)N)CN=C(N)N | ||
| Standard InChIKey | XUNKPNYCNUKOAU-VXJRNSOOSA-N | ||
| Standard InChI | InChI=1S/C54H110N36O10/c55-28(10-1-19-74-46(56)57)37(91)83-29(11-2-20-75-47(58)59)38(92)84-30(12-3-21-76-48(60)61)39(93)85-31(13-4-22-77-49(62)63)40(94)86-32(14-5-23-78-50(64)65)41(95)87-33(15-6-24-79-51(66)67)42(96)88-34(16-7-25-80-52(68)69)43(97)89-35(17-8-26-81-53(70)71)44(98)90-36(45(99)100)18-9-27-82-54(72)73/h28-36H,1-27,55H2,(H,83,91)(H,84,92)(H,85,93)(H,86,94)(H,87,95)(H,88,96)(H,89,97)(H,90,98)(H,99,100)(H4,56,57,74)(H4,58,59,75)(H4,60,61,76)(H4,62,63,77)(H4,64,65,78)(H4,66,67,79)(H4,68,69,80)(H4,70,71,81)(H4,72,73,82)/t28-,29-,30-,31-,32-,33-,34-,35-,36-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | (Arg)9 peptide is a cell-permeable peptide used for drug delivery. |

(Arg)9 peptide Dilution Calculator

(Arg)9 peptide Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
(Arg)9 (Nona-L-arginine;Peptide R9) is a cell-penetrating peptide; exhibits neuroprotective activity with an IC50 of 0.78 μM in the glutamic acid model. Sequence: Arg-Arg-Arg-Arg-Arg-Arg-Arg-Arg-Arg.
In Vitro:Poly-arginine (e.g. (Arg)9) and arginine-rich peptides (e.g. TAT, penetratin), which belong to a class of peptides with cell-penetrating properties are neuroprotective. (Arg)9 provides significant neuroprotection in a dose–response manner following glutamic acid exposure (IC50=0.78 μM). Following kainic acid exposure, (Arg)9 is neuroprotective, but less effective than in the glutamic acid model (IC50=0.81 μM). (Arg)9 also shows neuroprotection following in vitro ischemia (IC50=6 μM)[1].
In Vivo:(Arg)9) (D-isoform) is neuroprotective in rat stroke models. (Arg)9) is highly neuroprotective, with efficacy increasing with increasing arginine content, has the capacity to reduce glutamic acid-induced neuronal calcium influx and requires heparan sulfate preotoglycan-mediated endocytosis to induce a neuroprotective effect[2]. (Arg)9) (D-isoform) administered intravenously at a dose of 1000 nmol/kg 30 min after permanent middle cerebral artery occlusion (MCAO) reduces infarct volume[3].
References:
[1]. Meloni BP, et al. The neuroprotective efficacy of cell-penetrating peptides TAT, penetratin, Arg-9, and Pep-1 in glutamic acid, kainic acid, and in vitro ischemia injury models using primary cortical neuronal cultures. Cell Mol Neurobiol. 2014 Mar;34(2):173-81.
[2]. Meloni BP, et al. Poly-arginine and arginine-rich peptides are neuroprotective in stroke models. J Cereb Blood Flow Metab. 2015 Jun;35(6):993-1004.
[3]. Milani D, et al. Poly-arginine peptides reduce infarct volume in a permanent middle cerebral artery rat strokemodel. BMC Neurosci. 2016 May 3;17(1):19.
- SAR131675
Catalog No.:BCC5097
CAS No.:1433953-83-3
- Naratriptan
Catalog No.:BCC5053
CAS No.:143388-64-1
- Bryostatin 3
Catalog No.:BCC5620
CAS No.:143370-84-7
- SMIP004
Catalog No.:BCC1955
CAS No.:143360-00-3
- RS 56812 hydrochloride
Catalog No.:BCC6877
CAS No.:143339-12-2
- UNC 2400
Catalog No.:BCC5625
CAS No.:1433200-49-7
- Ac-YVAD-CHO
Catalog No.:BCC4021
CAS No.:143313-51-3
- H-Chg-OH
Catalog No.:BCC3162
CAS No.:14328-51-9
- RA-XI
Catalog No.:BCN3514
CAS No.:143277-27-4
- AGI-6780
Catalog No.:BCC1331
CAS No.:1432660-47-3
- LDN-212854
Catalog No.:BCC5330
CAS No.:1432597-26-6
- Tin protoporphyrin IX dichloride
Catalog No.:BCC6776
CAS No.:14325-05-4
- Poriol
Catalog No.:BCN6816
CAS No.:14348-16-4
- Cnidilin
Catalog No.:BCN2731
CAS No.:14348-22-2
- 5,8-Dihydroxypsoralen
Catalog No.:BCC8104
CAS No.:14348-23-3
- LOE 908 hydrochloride
Catalog No.:BCC7327
CAS No.:143482-60-4
- Emtricitabine
Catalog No.:BCC3774
CAS No.:143491-57-0
- Dammarenediol II
Catalog No.:BCN6240
CAS No.:14351-29-2
- 3-Acetoxy-24-hydroxydammara-20,25-diene
Catalog No.:BCN1569
CAS No.:143519-04-4
- (4S,5R)-3-(tert-Butoxycarbonyl)-2,2-dimethyl-4-phenyloxazolidine-5-carboxylic acid
Catalog No.:BCN8364
CAS No.:143527-70-2
- Shancigusin I
Catalog No.:BCN8272
CAS No.:1435488-35-9
- XMD17-109
Catalog No.:BCC2061
CAS No.:1435488-37-1
- L 012 sodium salt
Catalog No.:BCC6362
CAS No.:143556-24-5
- Rocuronium
Catalog No.:BCC1906
CAS No.:143558-00-3
Design of specific peptide inhibitors for group I phospholipase A2: structure of a complex formed between phospholipase A2 from Naja naja sagittifera (group I) and a designed peptide inhibitor Val-Ala-Phe-Arg-Ser (VAFRS) at 1.9 A resolution reveals unique features.[Pubmed:14529280]
Biochemistry. 2003 Oct 14;42(40):11701-6.
Phospholipase A(2) (PLA(2)) (E. C. 3.1.1.4) is a common enzyme in the two-way cascade mechanism leading to the production of proinflammatory compounds known as eicosanoids. The binding of phospholipase A(2) to the membrane surface and hydrolysis of phospholipids are thought to involve the formation of a hydrophobic channel into which a single substrate molecule diffuses before its cleavage. To regulate the production of proinflammatory compounds, a specific peptide inhibitor Val-Ala-Phe-Arg-Ser (VAFRS) for the group I PLA(2) enzymes has been designed and synthesized. PLA(2) was isolated from Indian cobra (Naja naja sagittifera) venom and purified to homogeneity. The binding studies indicated the K(i) value of 1.02 +/- 0.10 x 10(-8) M. The purified PLA(2) samples and the designed inhibitor VAFRS were cocrystallized. The crystal structure of the complex was determined and refined to 1.9 A resolution. The peptide binds to PLA(2) at the active site and fills the hydrophobic channel completely. However, its placement with respect to the channel is in the opposite direction as compared to those observed in group II PLA(2)'s. Furthermore, the predominant intermolecular interactions involve strong electrostatic interactions between the side chains of peptide Arg and Asp 49 of PLA(2) together with a number of van der Waals interactions with other residues. A good number of observed interactions between the peptide and the protein indicate the significance of a structure-based drug design approach. The novel factor in the present sequence of the peptide is related to the introduction of a positively charged residue at the C-terminal part of the peptide.
Structure-activity relationship of cyclic peptide penta-c[Asp-His(6)-DPhe(7)-Arg(8)-Trp(9)-Lys]-NH(2) at the human melanocortin-1 and -4 receptors: His(6) substitution.[Pubmed:12657270]
Bioorg Med Chem Lett. 2003 Apr 7;13(7):1307-11.
A series of MT-II related cyclic peptides, based on potent but non-selective hMC4R agonist (Penta-c[Asp-His(6)-DPhe(7)-Arg(8)-Trp(9)-Lys]-NH(2)) was prepared in which His(6) residue was systematically substituted. Two of the most interesting peptides identified in this study are Penta-c[Asp-5-ClAtc-DPhe-Arg-Trp-Lys]-NH(2) and Penta-c[Asp-5-ClAtc-DPhe-Cit-Trp-Lys]-NH(2) which are potent hMC4R agonists and are either inactive or weak partial agonists (not tested for their antagonist activities) in hMC1R, hMC3R and hMC5R agonist assays.
Fluorescence detection of MMP-9. I. MMP-9 selectively cleaves Lys-Gly-Pro-Arg-Ser-Leu-Ser-Gly-Lys peptide.[Pubmed:21446907]
Curr Pharm Biotechnol. 2011 May;12(5):834-8.
MMP-9 enzyme recognizes a peptide sequence Lys-Gly-Pro-Arg-Ser-Leu-Ser-Gly-Lys and cleaves the peptide into two parts. We synthesized a dual fluorophore beacon consisting of 5-FAM and Cy5 dyes. The fluorescence emission of the fluorescein moiety is dramatically quenched by Cy5 molecule due to Forster Resonance Energy Transfer (FRET) and the fluorescence of Cy5 is strongly enhanced. Upon addition of MMP-9 enzyme, the fluorescence of 5-FAM intensifies and Cy5 decreases. The control MMP-2 enzyme does not cause any changes in either 5-FAM or Cy5 fluorescence. We believe that our observation will help in early detection of elevated MMP-9 levels under disease conditions.
Purification, structural characterization, and myotropic activity of a peptide related to des-Arg(9)-bradykinin from an elasmobranch fish, the little skate, Leucoraja erinacea.[Pubmed:18502540]
Peptides. 2008 Aug;29(8):1280-6.
A bradykinin (BK)-related peptide was isolated from heat-denaturated plasma from an elasmobranch fish, the little skate, Leucoraja erinacea after incubation with porcine pancreatic kallikrein. The primary structure of the peptide (H-Gly-Ile-Thr-Ser-Trp-Leu-Pro-Phe-OH; skate BK) shows limited structural similarity to the mammalian B1 receptor agonist, des-Arg(9)-BK. The myotropic activities of synthetic skate BK, and the analog skate [Arg(9)]BK, were examined in isolated skate vascular and intestinal smooth muscle preparations. Skate BK produced a concentration-dependent constriction of the mesenteric artery (EC(50)=4.37x10(-8)M; maximum response=103.4+/-10.23% of the response to 60mM KCl) but the response to skate [Arg(9)]BK was appreciably weaker (response to 10(-6)M=73.0+/-23.4% of the response to 60mM KCl). Neither the first branchial gill arch nor the ventral aorta responded to either purified peptide. Skate BK also produced a concentration-dependent constriction of intestinal smooth muscle preparations (EC(50)=2.74x10(-7)M; maximum response 31.0+/-12.2% of the response to 10(-5)M acetylcholine). Skate [Arg(9)]BK was without effect on the intestinal preparation. The data provide evidence for the existence of the kallikrein-kinin system in a phylogenetically ancient vertebrate group and the greater potency of skate BK compared with the analog skate [Arg(9)]BK suggests that the receptor mediating vascular responses resembles the mammalian B1 receptor more closely than the B2 receptor.


