SMIP004Apoptosis inducer;downre CAS# 143360-00-3 |
- 3,3'-Diindolylmethane
Catalog No.:BCC1306
CAS No.:1968-05-4
- BAM7
Catalog No.:BCC1397
CAS No.:331244-89-4
- Bendamustine HCl
Catalog No.:BCC1153
CAS No.:3543-75-7
- Betulinic acid
Catalog No.:BCN5524
CAS No.:472-15-1
- Brassinolide
Catalog No.:BCC1438
CAS No.:72962-43-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 143360-00-3 | SDF | Download SDF |
| PubChem ID | 2747581 | Appearance | Powder |
| Formula | C13H19NO | M.Wt | 205.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (487.09 mM) *"≥" means soluble, but saturation unknown. | ||
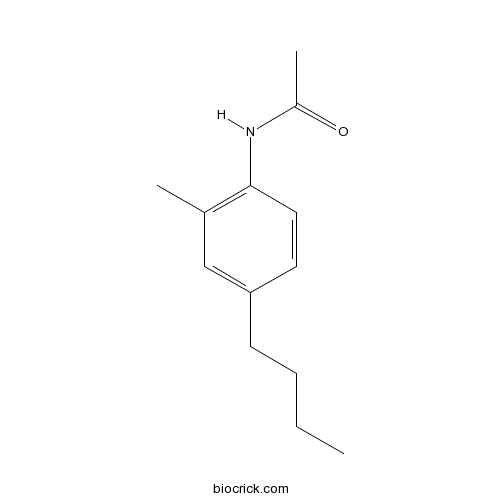
| Chemical Name | N-(4-butyl-2-methylphenyl)acetamide | ||
| SMILES | CCCCC1=CC(=C(C=C1)NC(=O)C)C | ||
| Standard InChIKey | ZFVMECVBUGMWIX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H19NO/c1-4-5-6-12-7-8-13(10(2)9-12)14-11(3)15/h7-9H,4-6H2,1-3H3,(H,14,15) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | SMIP004 is a novel inducer of cancer-cell selective apoptosis of human prostate cancer cells, it was found to downregulate SKP2 and to stabilize p27.
IC50 Value: 1.09 uM (MTT assay in LNCaP-S14 cells) [1]
Target: Apoptosis inducer; SKP2
in vitro: Whereas SMIP012 and 016 were moderately toxic in normal fibroblasts, SMIPs 001 and 004 showed substantial cancer cell specificity being at least five times more potent in LNCaP-S14 than in IMR90 cells , treatment with either MG132 or SMIP004 increased p27 half-life to > 6 h [1]. Both SMIP001 and 004 led to a strong increase in the recruitment of p27 to CDK2, while SMIP001 also slightly increased coprecipitation of p21 (Figure 6c). SMIP004 also reduced the amounts of cyclins E and A retrieved with CDK2. This was paralleled by a marked downregulation of cyclins E and A upon SMIP004 treatment. SMIP004 decreased the levels of positive cell cycle regulators, upregulated cyclin-dependent kinase inhibitors, and resulted in G1 arrest, inhibition of colony formation in soft agar, and cell death [2].
in vivo: SMIP004 potently inhibits the growth of prostate and breast cancer xenografts in mice [2].
Clinical trial: References: | |||||

SMIP004 Dilution Calculator

SMIP004 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.8709 mL | 24.3546 mL | 48.7092 mL | 97.4184 mL | 121.773 mL |
| 5 mM | 0.9742 mL | 4.8709 mL | 9.7418 mL | 19.4837 mL | 24.3546 mL |
| 10 mM | 0.4871 mL | 2.4355 mL | 4.8709 mL | 9.7418 mL | 12.1773 mL |
| 50 mM | 0.0974 mL | 0.4871 mL | 0.9742 mL | 1.9484 mL | 2.4355 mL |
| 100 mM | 0.0487 mL | 0.2435 mL | 0.4871 mL | 0.9742 mL | 1.2177 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50 Value: 1.09 uM (MTT assay in LNCaP-S14 cells) [1] SMIP004 (N-(4-butyl-2-methyl-phenyl) acetamide) is a novel inducer of cancer-cell selective apoptosis of human prostate cancer cells. Unlike SMIP001, SMIP004 was found to downregulate SKP2 and to stabilize p27, although neither SMIP is a proteasome inhibitor. in vitro: Whereas SMIP012 and 016 were moderately toxic in normal fibroblasts, SMIPs 001 and 004 showed substantial cancer cell specificity being at least five times more potent in LNCaP-S14 than in IMR90 cells , treatment with either MG132 or SMIP004 increased p27 half-life to > 6 h [1]. Both SMIP001 and 004 led to a strong increase in the recruitment of p27 to CDK2, while SMIP001 also slightly increased coprecipitation of p21 (Figure 6c). SMIP004 also reduced the amounts of cyclins E and A retrieved with CDK2. This was paralleled by a marked downregulation of cyclins E and A upon SMIP004 treatment. SMIP004 decreased the levels of positive cell cycle regulators, upregulated cyclin-dependent kinase inhibitors, and resulted in G1 arrest, inhibition of colony formation in soft agar, and cell death [2]. in vivo: SMIP004 potently inhibits the growth of prostate and breast cancer xenografts in mice [2]. Clinical trial: N/A
- RS 56812 hydrochloride
Catalog No.:BCC6877
CAS No.:143339-12-2
- UNC 2400
Catalog No.:BCC5625
CAS No.:1433200-49-7
- Ac-YVAD-CHO
Catalog No.:BCC4021
CAS No.:143313-51-3
- H-Chg-OH
Catalog No.:BCC3162
CAS No.:14328-51-9
- RA-XI
Catalog No.:BCN3514
CAS No.:143277-27-4
- AGI-6780
Catalog No.:BCC1331
CAS No.:1432660-47-3
- LDN-212854
Catalog No.:BCC5330
CAS No.:1432597-26-6
- Tin protoporphyrin IX dichloride
Catalog No.:BCC6776
CAS No.:14325-05-4
- Microstegiol
Catalog No.:BCN3157
CAS No.:143246-41-7
- (-)-Isolariciresinol 9'-O-glucoside
Catalog No.:BCN7708
CAS No.:143236-04-8
- (-)-Lyoniresinol 9'-O-glucoside
Catalog No.:BCN7037
CAS No.:143236-02-6
- Z-2-Nal-OH
Catalog No.:BCC3291
CAS No.:143218-10-4
- Bryostatin 3
Catalog No.:BCC5620
CAS No.:143370-84-7
- Naratriptan
Catalog No.:BCC5053
CAS No.:143388-64-1
- SAR131675
Catalog No.:BCC5097
CAS No.:1433953-83-3
- (Arg)9 peptide
Catalog No.:BCC5336
CAS No.:143413-47-2
- Poriol
Catalog No.:BCN6816
CAS No.:14348-16-4
- Cnidilin
Catalog No.:BCN2731
CAS No.:14348-22-2
- 5,8-Dihydroxypsoralen
Catalog No.:BCC8104
CAS No.:14348-23-3
- LOE 908 hydrochloride
Catalog No.:BCC7327
CAS No.:143482-60-4
- Emtricitabine
Catalog No.:BCC3774
CAS No.:143491-57-0
- Dammarenediol II
Catalog No.:BCN6240
CAS No.:14351-29-2
- 3-Acetoxy-24-hydroxydammara-20,25-diene
Catalog No.:BCN1569
CAS No.:143519-04-4
- (4S,5R)-3-(tert-Butoxycarbonyl)-2,2-dimethyl-4-phenyloxazolidine-5-carboxylic acid
Catalog No.:BCN8364
CAS No.:143527-70-2
Chemical genetics approach to restoring p27Kip1 reveals novel compounds with antiproliferative activity in prostate cancer cells.[Pubmed:21182779]
BMC Biol. 2010 Dec 23;8:153.
BACKGROUND: The cyclin-dependent kinase (CDK) inhibitor p27(Kip)(1) is downregulated in a majority of human cancers due to ectopic proteolysis by the ubiquitin-proteasome pathway. The expression of p27 is subject to multiple mechanisms of control involving several transcription factors, kinase pathways and at least three different ubiquitin ligases (SCF(SKP)(2), KPC, Pirh2), which regulate p27 transcription, translation, protein stability and subcellular localization. Using a chemical genetics approach, we have asked whether this control network can be modulated by small molecules such that p27 protein expression is restored in cancer cells. RESULTS: We developed a cell-based assay for measuring the levels of endogenous nuclear p27 in a high throughput screening format employing LNCaP prostate cancer cells engineered to overexpress SKP2. The assay platform was optimized to Z' factors of 0.48 - 0.6 and piloted by screening a total of 7368 chemical compounds. During the course of this work, we discovered two small molecules of previously unknown biological activity, SMIP001 and SMIP004, which increase the nuclear level of p27 at low micromolar concentrations. SMIPs (small molecule inhibitors of p27 depletion) also upregulate p21(Cip)(1), inhibit cellular CDK2 activity, induce G1 delay, inhibit colony formation in soft agar and exhibit preferential cytotoxicity in LNCaP cells relative to normal human fibroblasts. Unlike SMIP001, SMIP004 was found to downregulate SKP2 and to stabilize p27, although neither SMIP is a proteasome inhibitor. Whereas the screening endpoint - nuclear p27 - was robustly modulated by the compounds, SMIP-mediated cell cycle arrest and apoptosis were not strictly dependent on p27 and p21 - a finding that is explained by parallel inhibitory effects of SMIPs on positive cell cycle regulators, including cyclins E and A, and CDK4. CONCLUSIONS: Our data provide proof-of-principle that the screening platform we developed, using endogenous nuclear p27 as an endpoint, presents an effective means of identifying bioactive molecules with cancer selective antiproliferative activity. This approach, when applied to larger and more diverse sets of compounds with refined drug-like properties, bears the potential of revealing both unknown cellular pathways globally impinging on p27 and novel leads for chemotherapeutics targeting a prominent molecular defect of human cancers.
Small molecule-induced mitochondrial disruption directs prostate cancer inhibition via UPR signaling.[Pubmed:23902736]
Oncotarget. 2013 Aug;4(8):1212-29.
We previously identified SMIP004 (N-(4-butyl-2-methyl-phenyl) acetamide) as a novel inducer of cancer-cell selective apoptosis of human prostate cancer cells. SMIP004 decreased the levels of positive cell cycle regulators, upregulated cyclin-dependent kinase inhibitors, and resulted in G1 arrest, inhibition of colony formation in soft agar, and cell death. However, the mechanism of SMIP004-induced cancer cell selective apoptosis remained unknown. Here, we used chemical genomic and proteomic profiling to unravel a SMIP004-induced pro-apoptotic pathway, which initiates with disruption of mitochondrial respiration leading to oxidative stress. This, in turn, activates two pathways, one eliciting cell cycle arrest by rapidly targeting cyclin D1 for proteasomal degradation and driving the transcriptional downregulation of the androgen receptor, and a second pathway that activates pro-apoptotic signaling through MAPK activation downstream of the unfolded protein response (UPR). SMIP004 potently inhibits the growth of prostate and breast cancer xenografts in mice. Our data suggest that SMIP004, by inducing mitochondrial ROS formation, targets specific sensitivities of prostate cancer cells to redox and bioenergetic imbalances that can be exploited in cancer therapy.


