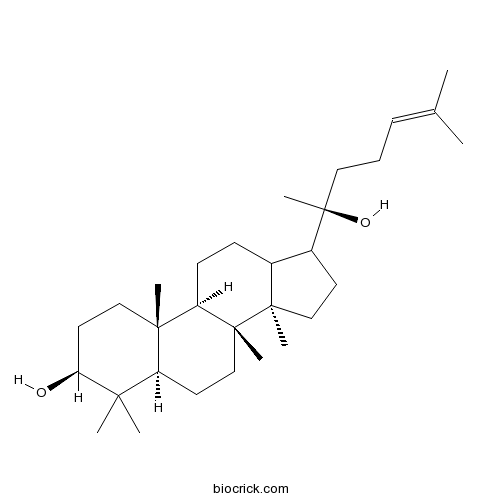
Dammarenediol IICAS# 14351-29-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 14351-29-2 | SDF | Download SDF |
| PubChem ID | 122880 | Appearance | Powder |
| Formula | C30H52O2 | M.Wt | 444.7 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3S,5R,8R,9R,10R,14R)-17-[(2S)-2-hydroxy-6-methylhept-5-en-2-yl]-4,4,8,10,14-pentamethyl-2,3,5,6,7,9,11,12,13,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol | ||
| SMILES | CC(=CCCC(C)(C1CCC2(C1CCC3C2(CCC4C3(CCC(C4(C)C)O)C)C)C)O)C | ||
| Standard InChIKey | NLHQJXWYMZLQJY-AXVVUFHWSA-N | ||
| Standard InChI | InChI=1S/C30H52O2/c1-20(2)10-9-16-30(8,32)22-13-18-28(6)21(22)11-12-24-27(5)17-15-25(31)26(3,4)23(27)14-19-29(24,28)7/h10,21-25,31-32H,9,11-19H2,1-8H3/t21?,22?,23-,24+,25-,27-,28+,29+,30-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Dammarenediol II synthase is a key rate-limiting enzyme during the process of rice synthesizes dammarane-type ginsenosides. 2. Dammarenediol II can inhibit vascular endothelial growth factor (VEGF)-induced intracellular reactive oxygen species generation and stress fiber formation and vascular endothelial-cadherin disruption, suggests that the natural drug dammarenediol II may have the ability to prevent diabetic microvascular complications, including diabetic retinopathy. 3. The medicinally important dammarenediol II can be ectopically produced in tobacco, and the production of dammarenediol-II in tobacco plants allows them to adopt a viral defense system. |
| Targets | VEGFR | Calcium Channel |

Dammarenediol II Dilution Calculator

Dammarenediol II Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2487 mL | 11.2435 mL | 22.4871 mL | 44.9741 mL | 56.2177 mL |
| 5 mM | 0.4497 mL | 2.2487 mL | 4.4974 mL | 8.9948 mL | 11.2435 mL |
| 10 mM | 0.2249 mL | 1.1244 mL | 2.2487 mL | 4.4974 mL | 5.6218 mL |
| 50 mM | 0.045 mL | 0.2249 mL | 0.4497 mL | 0.8995 mL | 1.1244 mL |
| 100 mM | 0.0225 mL | 0.1124 mL | 0.2249 mL | 0.4497 mL | 0.5622 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Emtricitabine
Catalog No.:BCC3774
CAS No.:143491-57-0
- LOE 908 hydrochloride
Catalog No.:BCC7327
CAS No.:143482-60-4
- 5,8-Dihydroxypsoralen
Catalog No.:BCC8104
CAS No.:14348-23-3
- Cnidilin
Catalog No.:BCN2731
CAS No.:14348-22-2
- Poriol
Catalog No.:BCN6816
CAS No.:14348-16-4
- (Arg)9 peptide
Catalog No.:BCC5336
CAS No.:143413-47-2
- SAR131675
Catalog No.:BCC5097
CAS No.:1433953-83-3
- Naratriptan
Catalog No.:BCC5053
CAS No.:143388-64-1
- Bryostatin 3
Catalog No.:BCC5620
CAS No.:143370-84-7
- SMIP004
Catalog No.:BCC1955
CAS No.:143360-00-3
- RS 56812 hydrochloride
Catalog No.:BCC6877
CAS No.:143339-12-2
- UNC 2400
Catalog No.:BCC5625
CAS No.:1433200-49-7
- 3-Acetoxy-24-hydroxydammara-20,25-diene
Catalog No.:BCN1569
CAS No.:143519-04-4
- (4S,5R)-3-(tert-Butoxycarbonyl)-2,2-dimethyl-4-phenyloxazolidine-5-carboxylic acid
Catalog No.:BCN8364
CAS No.:143527-70-2
- Shancigusin I
Catalog No.:BCN8272
CAS No.:1435488-35-9
- XMD17-109
Catalog No.:BCC2061
CAS No.:1435488-37-1
- L 012 sodium salt
Catalog No.:BCC6362
CAS No.:143556-24-5
- Rocuronium
Catalog No.:BCC1906
CAS No.:143558-00-3
- Virgatic acid
Catalog No.:BCN6744
CAS No.:14356-51-5
- Diprenorphine
Catalog No.:BCC5954
CAS No.:14357-78-9
- H-DL-Asp(OMe)-OMe.HCl
Catalog No.:BCC2901
CAS No.:14358-33-9
- A 419259 trihydrochloride
Catalog No.:BCC4308
CAS No.:1435934-25-0
- Cyclo(Leu-Leu)
Catalog No.:BCN2433
CAS No.:1436-27-7
- 5-Hydroxy-2-(4-methoxyphenyl)-8,8-dimethyl-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxypyrano[2,3-h]chromen-4-one
Catalog No.:BCC8807
CAS No.:143601-07-4
Dammarenediol-II Prevents VEGF-Mediated Microvascular Permeability in Diabetic Mice.[Pubmed:26400610]
Phytother Res. 2015 Dec;29(12):1910-6.
Diabetic retinopathy is a major diabetic complication predominantly caused by vascular endothelial growth factor (VEGF)-induced vascular permeability in the retina; however, treatments targeting glycemic control have not been successful. Here, we investigated the protective effect of dammarenediol-II, a precursor of triterpenoid saponin biosynthesis, on VEGF-induced vascular leakage using human umbilical vein endothelial cells (HUVECs) and diabetic mice. We overproduced the compound in transgenic tobacco expressing Panax ginseng dammarenediol-II synthase gene and purified using column chromatography. Analysis of the purified compound using a gas chromatography-mass spectrometry system revealed identical retention time and fragmentation pattern to those of authentic standard dammarenediol-II. Dammarenediol-II inhibited VEGF-induced intracellular reactive oxygen species generation, but it had no effect on the levels of intracellular Ca(2+) in HUVECs. We also found that dammarenediol-II inhibited VEGF-induced stress fiber formation and vascular endothelial-cadherin disruption, both of which play critical roles in modulating endothelial permeability. Notably, microvascular leakage in the retina of diabetic mice was successfully inhibited by intravitreal dammarenediol-II injection. Our results suggest that the natural drug dammarenediol-II may have the ability to prevent diabetic microvascular complications, including diabetic retinopathy.
3-epi-Dammarenediol II 1.075 hydrate: a dammarane triterpene from the bark of Aglaia eximia.[Pubmed:23284420]
Acta Crystallogr Sect E Struct Rep Online. 2012 Nov 1;68(Pt 11):o3089-90.
The title dammarane tritepene, 3alpha,20(S)-dihy-droxy-dammar-24-ene, which crystallized out in a hydrated form, C(30)H(52)O(2).1.075H(2)O, was isolated from the Aglaia eximia bark. The three cyclo-hexane rings adopt chair conformations. The cyclo-pentane has an envelope conformation with the quaternary C at position 14 as the flap atom with the maximum deviation of 0.288 (2) A. The methyl-heptene side chain is disordered over two positions with 0.505 (1):0.495 (1) site occupancies and is axially attached with an (+)-syn-clinal conformation. The hydroxyl group at position 3 of dammarane is in a different conformation to the corresponding hydroxyl in Dammarenediol II. In the crystal, the dammarane and water mol-ecules are linked by O(Dammarane)-Hcdots, three dots, centeredO(water) and O(water)-Hcdots, three dots, centeredO(Dammarane) hydrogen bonds into a three-dimensional network.
Production of dammarane-type sapogenins in rice by expressing the dammarenediol-II synthase gene from Panax ginseng C.A. Mey.[Pubmed:26398795]
Plant Sci. 2015 Oct;239:106-14.
Ginsenosides are the main active ingredients in Chinese medicinal ginseng; 2,3-oxidosqualene is a precursor metabolite to ginsenosides that is present in rice. Because rice lacks a key rate-limiting enzyme (dammarenediol-II synthase, DS), rice cannot synthesize dammarane-type ginsenosides. In this study, the ginseng (Panax ginseng CA Mey.) DS gene (GenBank: AB265170.1) was transformed into rice using agrobacterium, and 64 rice transgenic plants were produced. The Transfer-DNA (T-DNA) insertion sites in homozygous lines of the T2 generation were determined by using high-efficiency thermal asymmetric interlaced PCR (hiTAIL-PCR) and differed in all tested lines. One to two copies of the T-DNA were present in each transformant, and real-time PCR and Western blotting showed that the transformed DS gene could be transcribed and highly expressed. High performance liquid chromatography (HPLC) analysis showed that the dammarane-type sapogenin 20(S)-protopanaxadiol (PPD) content was 0.35-0.59 mg/g dw and the dammarane-type sapogenin 20(S)-protopanaxatriol (PPT) content was 0.23-0.43 mg/g dw in the transgenic rice. LC/MS analysis confirmed production of PPD and PPT. These results indicate that a new "ginseng rice" germplasm containing dammarane-type sapogenins has been successfully developed by transforming the ginseng DS gene into rice.
Dammarenediol-II production confers TMV tolerance in transgenic tobacco expressing Panax ginseng dammarenediol-II synthase.[Pubmed:22102695]
Plant Cell Physiol. 2012 Jan;53(1):173-82.
Panax ginseng is one of the famous medicinal plants. Ginsenosides, a class of tetracyclic triterpene saponins, are mainly responsible for its pharmacological activity. Most ginsenosides are composed of dammarenediol-II aglycone with various sugar moieties. Dammarenediol-II synthase is the first enzyme in the biosynthesis of ginsenosides. Here, we report that transgenic tobacco expressing the P. ginseng dammarenediol-II synthase gene (PgDDS) produced dammarenediol-II, and conferred resistance to Tobacco mosaic virus (TMV). Upon infection with TMV, lesions developed more rapidly in transgenic tobacco plants, and their size was smaller than those of wild-type plants. Transgenic tobacco plants showed a low level of both the viral titer and mRNA accumulation of TMV coat protein (CP) compared with the wild type. The production of dammarenediol-II in transgenic tobacco stimulated the expression of tobacco pathogenesis-related genes (PR1 and PR2) under both virus-untreated and -treated conditions. When the leaves of wild-type plants were inoculated with a mixture of TMV and dammarenediol-II, the leaves exhibited a reduced viral concentration and TMV-CP expression than those receiving TMV treatment alone. When the leaves of P. ginseng were infected with TMV, transcription of PgDDS was significantly increased. Transgenic P. ginseng plants harboring a beta-glucuronidase (GUS) gene driven by the PgDDS promoter were constructed. The GUS expression was activated when the transgenic ginseng plants were treated with TMV. These results indicate that the medicinally important dammarenediol-II can be ectopically produced in tobacco, and the production of dammarenediol-II in tobacco plants allows them to adopt a viral defense system.


