INDYDyrk1A and Dyrk1B inhibitor, selective CAS# 1169755-45-6 |
- FK 3311
Catalog No.:BCC1576
CAS No.:116686-15-8
- Iguratimod
Catalog No.:BCC1641
CAS No.:123663-49-0
- Celecoxib
Catalog No.:BCC1099
CAS No.:169590-42-5
- Etoricoxib
Catalog No.:BCC1565
CAS No.:202409-33-4
- Ibuprofen Lysine
Catalog No.:BCC2547
CAS No.:57469-77-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1169755-45-6 | SDF | Download SDF |
| PubChem ID | 69538603 | Appearance | Powder |
| Formula | C12H13NO2S | M.Wt | 235.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
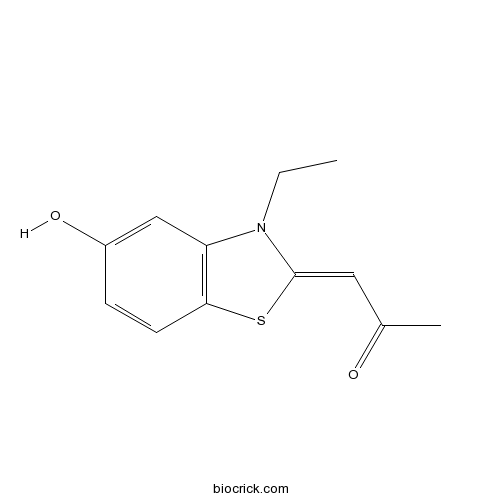
| Chemical Name | 1-(3-ethyl-5-hydroxy-1,3-benzothiazol-2-ylidene)propan-2-one | ||
| SMILES | CCN1C2=C(C=CC(=C2)O)SC1=CC(=O)C | ||
| Standard InChIKey | GCSZJMUFYOAHFY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H13NO2S/c1-3-13-10-7-9(15)4-5-11(10)16-12(13)6-8(2)14/h4-7,15H,3H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | DYRK1A/B inhibitor (IC50 values are 0.23 and 0.24 μM, for DYRK1B and DYRKA respectively). Binds at the ATP-binding cleft of the enzyme. Reverses aberrant tau-phosphorylation and rescues repressed calcineurin/NFAT signaling. Impairs the self-renewal capacity of subventricular zone neural stem cells. Also available as a prodrug, proINDY. |

INDY Dilution Calculator

INDY Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.2499 mL | 21.2495 mL | 42.4989 mL | 84.9979 mL | 106.2473 mL |
| 5 mM | 0.85 mL | 4.2499 mL | 8.4998 mL | 16.9996 mL | 21.2495 mL |
| 10 mM | 0.425 mL | 2.1249 mL | 4.2499 mL | 8.4998 mL | 10.6247 mL |
| 50 mM | 0.085 mL | 0.425 mL | 0.85 mL | 1.7 mL | 2.1249 mL |
| 100 mM | 0.0425 mL | 0.2125 mL | 0.425 mL | 0.85 mL | 1.0625 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
INDY is a selective inhibitor of Dyrk1A and Dyrk1B with IC50 values of 0.23 and 0.24 μM, respectively [1].
Dual-specificity tyrosine-(Y) -phosphorylation-regulated kinase 1A (Dyrk1A) is a serine/threonine kinase expressed in adult brains as well as fetal and phosphorylates myriad proteins. DYRK1B regulates nuclear functions mainly expressed in muscle and testis [1].
INDY is a selective Dyrk1A and Dyrk1B inhibitor. INDY inhibited Dyrk1A against ATP with Km and Ki of 37 and 0.18 μM, respectively. INDY (10 μM) exhibited > 90% inhibition on CLK1, CLK4, DYRK2, DYRK3, casein kinase 1 (CSNK1D) and PIM1. In COS7 cells, INDY (3 μM) inhibited tau-phosphorylation induced by Dyrk1A. In HEK293 cells, Dyrk1A inhibited the nuclear accumulation of NFATc1, while INDY relocated NFATc1 into the nucleus [1]. In EGFR-expressing glioblastomas tumor-initiating cells (GBM-TICs), INDY impaired cells self-renewal capacity and inhibited tumor growth and survival [2].
In X. laevis embryo overexpressed xDyrk1A, deformity was observed in the head and the eye of stage 40/41 tadpoles. While proINDY rescued the morphological abnormalities [1].
References:
[1]. Ogawa Y, Nonaka Y, Goto T, et al. Development of a novel selective inhibitor of the Down syndrome-related kinase Dyrk1A. Nat Commun, 2010, 1: 86.
[2]. Pozo N, Zahonero C, Fernández P, et al. Inhibition of DYRK1A destabilizes EGFR and reduces EGFR-dependent glioblastoma growth. J Clin Invest, 2013, 123(6): 2475-2487.
- XL413 hydrochloride
Catalog No.:BCC4039
CAS No.:1169562-71-3
- XL413
Catalog No.:BCC4241
CAS No.:1169558-38-6
- Monohydroxyisoaflavinine
Catalog No.:BCN7284
CAS No.:116865-09-9
- 20-Hydroxyaflavinine
Catalog No.:BCN7283
CAS No.:116865-08-8
- Fmoc-D-Ser-OH
Catalog No.:BCC3547
CAS No.:116861-26-8
- 5-Formamide-1-(2-formyloxyethl)pyrazole
Catalog No.:BCC8747
CAS No.:116856-18-9
- GDC-0623
Catalog No.:BCC4150
CAS No.:1168091-68-6
- 4',5,6,7-Tetramethoxyflavone
Catalog No.:BCN8256
CAS No.:1168-42-9
- 2-(4-Hydroxyphenyl)-6-methyl-2,3-dihydro-4H-pyran-4-one
Catalog No.:BCN1610
CAS No.:1167483-18-2
- 9'''-Methyl salvianolate B
Catalog No.:BCN2923
CAS No.:1167424-32-9
- 9'-Methyl lithospermate B
Catalog No.:BCN2824
CAS No.:1167424-31-8
- Novaluron
Catalog No.:BCC5466
CAS No.:116714-46-6
- Sculponeatin N
Catalog No.:BCN6044
CAS No.:1169805-98-4
- Sculponeatin O
Catalog No.:BCN6045
CAS No.:1169806-00-1
- Sculponeatic acid
Catalog No.:BCN6046
CAS No.:1169806-02-3
- Rubiadin
Catalog No.:BCN6047
CAS No.:117-02-2
- Dantron
Catalog No.:BCN6048
CAS No.:117-10-2
- Quercetin
Catalog No.:BCN6049
CAS No.:117-39-5
- 2-Amino-3-hydroxyanthraquinone
Catalog No.:BCC8527
CAS No.:117-77-1
- 2-Anthraquinonecarboxylic acid
Catalog No.:BCN3451
CAS No.:117-78-2
- Bis(2-ethylhexyl) phthalate
Catalog No.:BCN6054
CAS No.:117-81-7
- Boc-Asp(Ofm)-OH
Catalog No.:BCC3366
CAS No.:117014-32-1
- Anwuweizonic acid
Catalog No.:BCN3633
CAS No.:117020-59-4
- Licopyranocoumarin
Catalog No.:BCN7900
CAS No.:117038-80-9
Increased mitochondrial biogenesis preserves intestinal stem cell homeostasis and contributes to longevity in Indy mutant flies.[Pubmed:24827528]
Aging (Albany NY). 2014 Apr;6(4):335-50.
The Drosophila INDY (I'm Not Dead Yet) gene encodes a plasma membrane transporter of Krebs cycle intermediates, with robust expression in tissues associated with metabolism. Reduced INDY alters metabolism and extends longevity in a manner similar to caloric restriction (CR); however, little is known about the tissue specific physiological effects of INDY reduction. Here we focused on the effects of INDY reduction in the Drosophila midgut due to the importance of intestinal tissue homeostasis in healthy aging and longevity. The expression of INDY mRNA in the midgut changes in response to aging and nutrition. Genetic reduction of INDY expression increases midgut expression of the mitochondrial regulator spargel/dPGC-1, which is accompanied by increased mitochondrial biogenesis and reduced reactive oxygen species (ROS). These physiological changes in the INDY mutant midgut preserve intestinal stem cell (ISC) homeostasis and are associated with healthy aging. Genetic studies confirm that dPGC-1 mediates the regulatory effects of INDY, as illustrated by lack of longevity extension and ISC homeostasis in flies with mutations in both INDY and dPGC1. Our data suggest INDY may be a physiological regulator that modulates intermediary metabolism in response to changes in nutrient availability and organismal needs by modulating dPGC-1.
The role of INDY in metabolism, health and longevity.[Pubmed:26106407]
Front Genet. 2015 Jun 9;6:204.
INDY (I'm Not Dead Yet) encodes the fly homolog of a mammalian SLC13A5 plasma membrane transporter. INDY is expressed in metabolically active tissues functioning as a transporter of Krebs cycle intermediates with the highest affinity for citrate. Decreased expression of the INDY gene extends longevity in Drosophila and C. elegans. Reduction of INDY or its respective homologs in C. elegans and mice induces metabolic and physiological changes similar to those observed in calorie restriction. It is thought that these physiological changes are due to altered levels of cytoplasmic citrate, which directly impacts Krebs cycle energy production as a result of shifts in substrate availability. Citrate cleavage is a key event during lipid and glucose metabolism; thus, reduction of citrate due to INDY reduction alters these processes. With regards to mammals, mice with reduced INDY (mINDY(-/-)) also exhibit changes in glucose metabolism, mitochondrial biogenesis and are protected from the negative effects of a high calorie diet. Together, these data support a role for INDY as a metabolic regulator, which suggests INDY as a therapeutic target for treatment of diet and age-related disorders such as Type II Diabetes and obesity.
The human longevity gene homolog INDY and interleukin-6 interact in hepatic lipid metabolism.[Pubmed:28133767]
Hepatology. 2017 Aug;66(2):616-630.
Reduced expression of the INDY ("I am Not Dead, Yet") gene in lower organisms promotes longevity in a manner akin to caloric restriction. Deletion of the mammalian homolog of INDY (mINDY, Slc13a5) encoding for a plasma membrane-associated citrate transporter expressed highly in the liver, protects mice from high-fat diet-induced and aging-induced obesity and hepatic fat accumulation through a mechanism resembling caloric restriction. We studied a possible role of mINDY in human hepatic fat metabolism. In obese, insulin-resistant patients with nonalcoholic fatty liver disease, hepatic mINDY expression was increased and mINDY expression was also independently associated with hepatic steatosis. In nonhuman primates, a 2-year high-fat, high-sucrose diet increased hepatic mINDY expression. Liver microarray analysis showed that high mINDY expression was associated with pathways involved in hepatic lipid metabolism and immunological processes. Interleukin-6 (IL-6) was identified as a regulator of mINDY by binding to its cognate receptor. Studies in human primary hepatocytes confirmed that IL-6 markedly induced mINDY transcription through the IL-6 receptor and activation of the transcription factor signal transducer and activator of transcription 3, and a putative start site of the human mINDY promoter was determined. Activation of the IL-6-signal transducer and activator of transcription 3 pathway stimulated mINDY expression, enhanced cytoplasmic citrate influx, and augmented hepatic lipogenesis in vivo. In contrast, deletion of mINDY completely prevented the stimulating effect of IL-6 on citrate uptake and reduced hepatic lipogenesis. These data show that mINDY is increased in liver of obese humans and nonhuman primates with NALFD. Moreover, our data identify mINDY as a target gene of IL-6 and determine novel functions of IL-6 through mINDY. CONCLUSION: Targeting human mINDY may have therapeutic potential in obese patients with nonalcoholic fatty liver disease. German Clinical Trials Register: DRKS00005450. (Hepatology 2017;66:616-630).
Knockdown of Indy/CeNac2 extends Caenorhabditis elegans life span by inducing AMPK/aak-2.[Pubmed:26318988]
Aging (Albany NY). 2015 Aug;7(8):553-67.
Reducing the expression of the INDY (Acronym for 'I'm Not Dead, Yet') gene in lower organisms promotes longevity and leads to a phenotype that resembles various aspects of caloric restriction. In C. elegans, the available data on life span extension is controversial. Therefore, the aim of this study was to determine the role of the C. elegans INDY homolog CeNAC2 in life span regulation and to delineate possible molecular mechanisms. siRNA against INDY/CeNAC2 was used to reduce expression of INDY/CeNAC2. Mean life span was assessed in four independent experiments, as well as whole body fat content and AMPK activation. Moreover, the effect of INDY/CeNAC2 knockdown in C. elegans with inactivating variants of AMPK (TG38) was studied. Knockdown of INDY/CeNAC2 increased life span by 22+/-3 % compared to control siRNA treated C. elegans, together with a decrease in whole body fat content by ~50%. INDY/CeNAC2 reduction also increased the activation of the intracellular energy sensor AMPK/aak2. In worms without functional AMPK/aak2, life span was not extended when INDY/CeNAC2 was reduced. Inhibition of glycolysis with deoxyglucose, an intervention known to increase AMPK/aak2 activity and life span, did not promote longevity when INDY/CeNAC2 was knocked down. Together, these data indicate that reducing the expression of INDY/CeNAC2 increases life span in C. elegans, an effect mediated at least in part by AMPK/aak2.
Inhibition of DYRK1A destabilizes EGFR and reduces EGFR-dependent glioblastoma growth.[Pubmed:23635774]
J Clin Invest. 2013 Jun;123(6):2475-87.
Glioblastomas (GBMs) are very aggressive tumors that are resistant to conventional chemo- and radiotherapy. New molecular therapeutic strategies are required to effectively eliminate the subpopulation of GBM tumor-initiating cells that are responsible for relapse. Since EGFR is altered in 50% of GBMs, it represents one of the most promising targets; however, EGFR kinase inhibitors have produced poor results in clinical assays, with no clear explanation for the observed resistance. We uncovered a fundamental role for the dual-specificity tyrosine phosphorylation-regulated kinase, DYRK1A, in regulating EGFR in GBMs. We found that DYRK1A was highly expressed in these tumors and that its expression was correlated with that of EGFR. Moreover, DYRK1A inhibition promoted EGFR degradation in primary GBM cell lines and neural progenitor cells, sharply reducing the self-renewal capacity of normal and tumorigenic cells. Most importantly, our data suggest that a subset of GBMs depends on high surface EGFR levels, as DYRK1A inhibition compromised their survival and produced a profound decrease in tumor burden. We propose that the recovery of EGFR stability is a key oncogenic event in a large proportion of gliomas and that pharmacological inhibition of DYRK1A could represent a promising therapeutic intervention for EGFR-dependent GBMs.
Development of a novel selective inhibitor of the Down syndrome-related kinase Dyrk1A.[Pubmed:20981014]
Nat Commun. 2010 Oct 5;1:86.
Dyrk1A (dual-specificity tyrosine-(Y)-phosphorylation-regulated kinase 1A) is a serine/threonine kinase essential for brain development and function, and its excessive activity is considered a pathogenic factor in Down syndrome. The development of potent, selective inhibitors of Dyrk1A would help to elucidate the molecular mechanisms of normal and diseased brains, and may provide a new lead compound for molecular-targeted drug discovery. Here, we report a novel Dyrk1A inhibitor, INDY, a benzothiazole derivative showing a potent ATP-competitive inhibitory effect with IC(50) and K(i) values of 0.24 and 0.18 muM, respectively. X-ray crystallography of the Dyrk1A/INDY complex revealed the binding of INDY in the ATP pocket of the enzyme. INDY effectively reversed the aberrant tau-phosphorylation and rescued the repressed NFAT (nuclear factor of activated T cell) signalling induced by Dyrk1A overexpression. Importantly, proINDY, a prodrug of INDY, effectively recovered Xenopus embryos from head malformation induced by Dyrk1A overexpression, resulting in normally developed embryos and demonstrating the utility of proINDY in vivo.


