XL413Cdc7 inhibitor CAS# 1169558-38-6 |
- WYE-354
Catalog No.:BCC1059
CAS No.:1062169-56-5
- GDC-mTOR inhibitor
Catalog No.:BCC1781
CAS No.:1207358-59-5
- GDC-0349
Catalog No.:BCC1094
CAS No.:1207360-89-1
- LY 303511
Catalog No.:BCC1715
CAS No.:154447-38-8
- Nordihydroguaiaretic acid
Catalog No.:BCC1805
CAS No.:500-38-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1169558-38-6 | SDF | Download SDF |
| PubChem ID | 57899889 | Appearance | Powder |
| Formula | C14H12ClN3O2 | M.Wt | 289.72 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
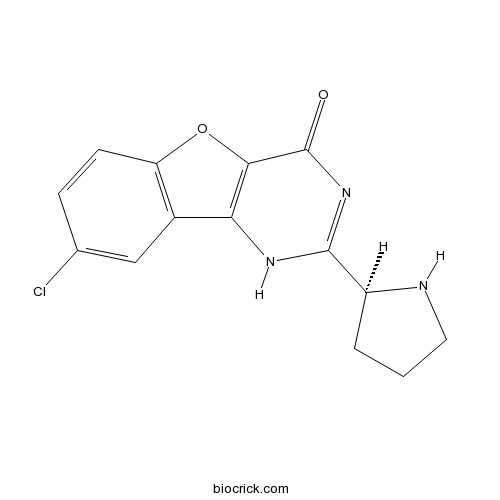
| Chemical Name | 8-chloro-2-[(2S)-pyrrolidin-2-yl]-1H-[1]benzofuro[3,2-d]pyrimidin-4-one | ||
| SMILES | C1CC(NC1)C2=NC(=O)C3=C(N2)C4=C(O3)C=CC(=C4)Cl | ||
| Standard InChIKey | JJWLXRKVUJDJKG-VIFPVBQESA-N | ||
| Standard InChI | InChI=1S/C14H12ClN3O2/c15-7-3-4-10-8(6-7)11-12(20-10)14(19)18-13(17-11)9-2-1-5-16-9/h3-4,6,9,16H,1-2,5H2,(H,17,18,19)/t9-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | XL413 is a potent, selective and ATP competitive inhibitor of Cdc7, with an IC50 of 3.4 nM, and also shows potent effect with IC50s of 215, 42 nM on CK2, PIM1, respectively, and an EC50 of 118 nM on pMCM.In Vitro:XL413 inhibits the cell proliferation (IC50 = 2685 nM), decreases cell viability (IC50 = 2142 nM) and elicits the caspase 3/7 activity (EC50 = 2288 nM) in Colo-205 cells. XL413 also significantly inhibits the anchorage-independent growth of colo-205 in soft agar (IC50 = 715 nM)[1]. XL413 shows cytotoxic effects on tumors, with IC50 of 22.9 µM in HCC1954 cells and 1.1 µM in Colo-205 cells. XL413 induces apoptosis in the Colo-205 cells, but not in HCC1954 cells. XL413 is effective DDK inhibitors in vitro, with IC50 of 22.7 nM. XL413 is defective in inhibiting DDK-dependent Mcm2 phosphorylation in HCC1954 cells but is effective in Colo-205 cells[2].In Vivo:XL413 (100 mg/kg, p.o.) shows excellent plasma exposures in mice and possesses good PK properties. XL413 (10, 30, or 100 mg/kg, p.o.) is well tolerated at all the doses, with no significant body weight loss[1]. References: | |||||

XL413 Dilution Calculator

XL413 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4516 mL | 17.258 mL | 34.5161 mL | 69.0322 mL | 86.2902 mL |
| 5 mM | 0.6903 mL | 3.4516 mL | 6.9032 mL | 13.8064 mL | 17.258 mL |
| 10 mM | 0.3452 mL | 1.7258 mL | 3.4516 mL | 6.9032 mL | 8.629 mL |
| 50 mM | 0.069 mL | 0.3452 mL | 0.6903 mL | 1.3806 mL | 1.7258 mL |
| 100 mM | 0.0345 mL | 0.1726 mL | 0.3452 mL | 0.6903 mL | 0.8629 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
XL413 is a potent and selective Cdc7 inhibitor with an IC50 of 3.7 nM, >60-fold selectivity against CK2, >10-fold selectivity against PIM, and >300-fold selectivity against a panel of over 100 protein kinases.
- Monohydroxyisoaflavinine
Catalog No.:BCN7284
CAS No.:116865-09-9
- 20-Hydroxyaflavinine
Catalog No.:BCN7283
CAS No.:116865-08-8
- Fmoc-D-Ser-OH
Catalog No.:BCC3547
CAS No.:116861-26-8
- 5-Formamide-1-(2-formyloxyethl)pyrazole
Catalog No.:BCC8747
CAS No.:116856-18-9
- GDC-0623
Catalog No.:BCC4150
CAS No.:1168091-68-6
- 4',5,6,7-Tetramethoxyflavone
Catalog No.:BCN8256
CAS No.:1168-42-9
- 2-(4-Hydroxyphenyl)-6-methyl-2,3-dihydro-4H-pyran-4-one
Catalog No.:BCN1610
CAS No.:1167483-18-2
- 9'''-Methyl salvianolate B
Catalog No.:BCN2923
CAS No.:1167424-32-9
- 9'-Methyl lithospermate B
Catalog No.:BCN2824
CAS No.:1167424-31-8
- Novaluron
Catalog No.:BCC5466
CAS No.:116714-46-6
- Glycyrrhisoflavone
Catalog No.:BCN2930
CAS No.:116709-70-7
- H-9 dihydrochloride
Catalog No.:BCC5656
CAS No.:116700-36-8
- XL413 hydrochloride
Catalog No.:BCC4039
CAS No.:1169562-71-3
- INDY
Catalog No.:BCC6349
CAS No.:1169755-45-6
- Sculponeatin N
Catalog No.:BCN6044
CAS No.:1169805-98-4
- Sculponeatin O
Catalog No.:BCN6045
CAS No.:1169806-00-1
- Sculponeatic acid
Catalog No.:BCN6046
CAS No.:1169806-02-3
- Rubiadin
Catalog No.:BCN6047
CAS No.:117-02-2
- Dantron
Catalog No.:BCN6048
CAS No.:117-10-2
- Quercetin
Catalog No.:BCN6049
CAS No.:117-39-5
- 2-Amino-3-hydroxyanthraquinone
Catalog No.:BCC8527
CAS No.:117-77-1
- 2-Anthraquinonecarboxylic acid
Catalog No.:BCN3451
CAS No.:117-78-2
- Bis(2-ethylhexyl) phthalate
Catalog No.:BCN6054
CAS No.:117-81-7
- Boc-Asp(Ofm)-OH
Catalog No.:BCC3366
CAS No.:117014-32-1
Discovery of XL413, a potent and selective CDC7 inhibitor.[Pubmed:22560567]
Bioorg Med Chem Lett. 2012 Jun 1;22(11):3727-31.
CDC7 is a serine/threonine kinase that has been shown to be required for the initiation and maintenance of DNA replication. Up-regulation of CDC7 is detected in multiple tumor cell lines, with inhibition of CDC7 resulting in cell cycle arrest. In this paper, we disclose the discovery of a potent and selective CDC7 inhibitor, XL413 (14), which was advanced into Phase 1 clinical trials. Starting from advanced lead 3, described in a preceding communication, we optimized the CDC7 potency and selectivity to demonstrate in vitro CDC7 dependent cell cycle arrest and in vivo tumor growth inhibition in a Colo-205 xenograft model.
The potent Cdc7-Dbf4 (DDK) kinase inhibitor XL413 has limited activity in many cancer cell lines and discovery of potential new DDK inhibitor scaffolds.[Pubmed:25412417]
PLoS One. 2014 Nov 20;9(11):e113300.
Cdc7-Dbf4 kinase or DDK (Dbf4-dependent kinase) is required to initiate DNA replication by phosphorylating and activating the replicative Mcm2-7 DNA helicase. DDK is overexpressed in many tumor cells and is an emerging chemotherapeutic target since DDK inhibition causes apoptosis of diverse cancer cell types but not of normal cells. PHA-767491 and XL413 are among a number of potent DDK inhibitors with low nanomolar IC50 values against the purified kinase. Although XL413 is highly selective for DDK, its activity has not been extensively characterized on cell lines. We measured anti-proliferative and apoptotic effects of XL413 on a panel of tumor cell lines compared to PHA-767491, whose activity is well characterized. Both compounds were effective biochemical DDK inhibitors but surprisingly, their activities in cell lines were highly divergent. Unlike PHA-767491, XL413 had significant anti-proliferative activity against only one of the ten cell lines tested. Since XL413 did not effectively inhibit DDK in multiple cell lines, this compound likely has limited bioavailability. To identify potential leads for additional DDK inhibitors, we also tested the cross-reactivity of approximately 400 known kinase inhibitors against DDK using a DDK thermal stability shift assay (TSA). We identified 11 compounds that significantly stabilized DDK. Several inhibited DDK with comparable potency to PHA-767491, including Chk1 and PKR kinase inhibitors, but had divergent chemical scaffolds from known DDK inhibitors. Taken together, these data show that several well-known kinase inhibitors cross-react with DDK and also highlight the opportunity to design additional specific, biologically active DDK inhibitors for use as chemotherapeutic agents.
XL413, a cell division cycle 7 kinase inhibitor enhanced the anti-fibrotic effect of pirfenidone on TGF-beta1-stimulated C3H10T1/2 cells via Smad2/4.[Pubmed:26589264]
Exp Cell Res. 2015 Dec 10;339(2):289-99.
Pirfenidone is an orally bioavailable synthetic compound with therapeutic potential for idiopathic pulmonary fibrosis. It is thought to act through antioxidant and anti-fibrotic pathways. Pirfenidone inhibits proliferation and/or myofibroblast differentiation of a wide range of cell types, however, little studies have analyzed the effect of pirfenidone on the mesenchymal stem cells, which play an important role on the origin of myofibroblasts. We recently found that pirfenidone had anti-proliferative activity via G1 phase arrest and cell division cycle 7 (Cdc7) kinase expression decrease in transforming growth factor-beta1 (TGF-beta1)-stimulated murine mesenchymal stem C3H10T1/2 cells. Pirfenidone also had inhibiting effect on the migration and alpha-SMA expression. Moreover, in this study we showed for the first time that Cdc7 inhibitor XL413 enhanced the anti-fibrotic activity of pirfenidone via depressed the expression of Smad2/4 proteins, and also prevented the nuclear accumulation and translocation of Smad2 protein. In conclusion, we demonstrated that pirfenidone inhibited proliferation, migration and differentiation of TGF-beta1-stimulated C3H10T1/2 cells, which could be enhanced by Cdc7 inhibitor XL413, via Smad2/4. Combination with pirfenidone and XL413 might provide a potential candidate for the treatment of TGF-beta1 associated fibrosis. It needs in vivo studies to further validate its therapeutic function and safety in the future.


