H-9 dihydrochlorideProtein kinase inhibitor CAS# 116700-36-8 |
- Hydroxyfasudil
Catalog No.:BCC1635
CAS No.:105628-72-6
- chroman 1
Catalog No.:BCC1480
CAS No.:1273579-40-0
- Y-27632 dihydrochloride
Catalog No.:BCC1273
CAS No.:129830-38-2
- Hydroxyfasudil hydrochloride
Catalog No.:BCC1636
CAS No.:155558-32-0
- H-1152 dihydrochloride
Catalog No.:BCC1616
CAS No.:871543-07-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 116700-36-8 | SDF | Download SDF |
| PubChem ID | 11957465 | Appearance | Powder |
| Formula | C11H15Cl2N3O2S | M.Wt | 324.22 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water and to 100 mM in DMSO | ||
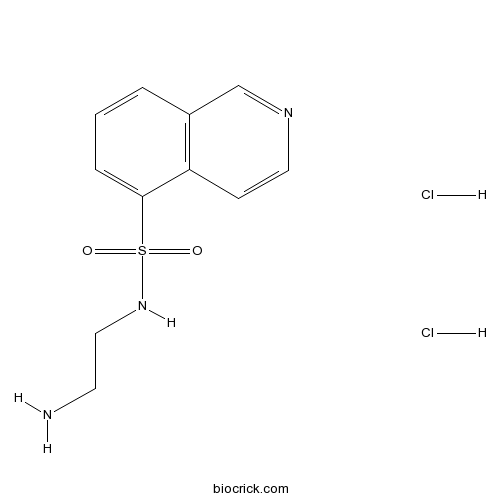
| Chemical Name | N-(2-aminoethyl)isoquinoline-5-sulfonamide;dihydrochloride | ||
| SMILES | C1=CC2=C(C=CN=C2)C(=C1)S(=O)(=O)NCCN.Cl.Cl | ||
| Standard InChIKey | HBLCYSFLYMHCBM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H13N3O2S.2ClH/c12-5-7-14-17(15,16)11-3-1-2-9-8-13-6-4-10(9)11;;/h1-4,6,8,14H,5,7,12H2;2*1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Protein kinase inhibitor. Inhibits PKA (Ki = 1.9 μM), PKG (Ki = 0.9 μM), CaMK II (Ki = 60 μM), PKC (Ki = 18 μM), casein kinase I (Ki = 110 μ M) and casein kinase II (Ki > 300 μM). |

H-9 dihydrochloride Dilution Calculator

H-9 dihydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0843 mL | 15.4216 mL | 30.8433 mL | 61.6865 mL | 77.1081 mL |
| 5 mM | 0.6169 mL | 3.0843 mL | 6.1687 mL | 12.3373 mL | 15.4216 mL |
| 10 mM | 0.3084 mL | 1.5422 mL | 3.0843 mL | 6.1687 mL | 7.7108 mL |
| 50 mM | 0.0617 mL | 0.3084 mL | 0.6169 mL | 1.2337 mL | 1.5422 mL |
| 100 mM | 0.0308 mL | 0.1542 mL | 0.3084 mL | 0.6169 mL | 0.7711 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- FK 3311
Catalog No.:BCC1576
CAS No.:116686-15-8
- AZD7687
Catalog No.:BCC1394
CAS No.:1166827-44-6
- Mycophenolate mofetil hydrochloride
Catalog No.:BCC4117
CAS No.:116680-01-4
- Mibefradil dihydrochloride
Catalog No.:BCC1749
CAS No.:116666-63-8
- Mibefradil
Catalog No.:BCC1748
CAS No.:116644-53-2
- A66
Catalog No.:BCC3715
CAS No.:1166227-08-2
- 3-Methylamino-1-(2-thienyl)-1-propanol
Catalog No.:BCC8636
CAS No.:116539-55-0
- Dehydroalisol B 23-acetate
Catalog No.:BCC9240
CAS No.:
- Aflatoxin G1
Catalog No.:BCC9214
CAS No.:1165-39-5
- 9alpha,13alpha-Epidioxyabiet-8(14)-en-18-oic acid
Catalog No.:BCN1611
CAS No.:116499-73-1
- 5,5'-Dimethoxylariciresinol
Catalog No.:BCN6043
CAS No.:116498-58-9
- ML 145
Catalog No.:BCC7876
CAS No.:1164500-72-4
- Glycyrrhisoflavone
Catalog No.:BCN2930
CAS No.:116709-70-7
- Novaluron
Catalog No.:BCC5466
CAS No.:116714-46-6
- 9'-Methyl lithospermate B
Catalog No.:BCN2824
CAS No.:1167424-31-8
- 9'''-Methyl salvianolate B
Catalog No.:BCN2923
CAS No.:1167424-32-9
- 2-(4-Hydroxyphenyl)-6-methyl-2,3-dihydro-4H-pyran-4-one
Catalog No.:BCN1610
CAS No.:1167483-18-2
- 4',5,6,7-Tetramethoxyflavone
Catalog No.:BCN8256
CAS No.:1168-42-9
- GDC-0623
Catalog No.:BCC4150
CAS No.:1168091-68-6
- 5-Formamide-1-(2-formyloxyethl)pyrazole
Catalog No.:BCC8747
CAS No.:116856-18-9
- Fmoc-D-Ser-OH
Catalog No.:BCC3547
CAS No.:116861-26-8
- 20-Hydroxyaflavinine
Catalog No.:BCN7283
CAS No.:116865-08-8
- Monohydroxyisoaflavinine
Catalog No.:BCN7284
CAS No.:116865-09-9
- XL413
Catalog No.:BCC4241
CAS No.:1169558-38-6
Evidence for a role for cyclic AMP in modulating the action of 5-HT and an excitatory neuropeptide, FLP17A, in the pharyngeal muscle of Caenorhabditis elegans.[Pubmed:18463910]
Invert Neurosci. 2008 Jun;8(2):91-100.
The feeding activity of the nematode Caenorhabditis elegans is regulated by an anatomically well-defined network of 20 enteric neurones that employs small molecule and neuropeptidergic signalling. Two of the most potent excitatory agents are 5-HT and the neuropeptide FLP17A. Here we have examined the role of cAMP in modulating their excitatory actions by pharmacological manipulation of the level of cAMP. Application of the membrane permeable cAMP analogue, dibutyryl-cAMP (1 microM), enhanced the excitatory response to both FLP17A and 5-HT. Furthermore, the adenylyl cyclase activator, forskolin (50 nM), significantly enhanced the excitatory response to both FLP17A and 5-HT. The phosphodiesterase inhibitor, ibudilast (10 microM), enhanced the excitatory response to FLP17A. The protein kinase inhibitor, H-9 dihydrochloride (10 microM) significantly reduced the excitatory response to 5-HT. H-9 dihydrochloride also had a direct effect on pharyngeal activity. The effect of FLP17A and 5-HT on two mutants, egl-8 (loss-of-function phospholipase-Cbeta) and egl-30 (loss-of-function Galphaq) was also investigated. Both these mutants have a lower pharyngeal pumping rate than wild-type which has to be considered when interpreting the effects of these mutations on the excitatory responses to FLP17A and 5HT. However, even taking into consideration the lower basal activity of these mutants, it is clear that the percentage increase in pharyngeal pumping rate induced by FLP17A is greatly reduced in both mutants compared to wild-type. In the case of 5-HT, the effect of the mutant backgrounds on the response was less pronounced. Overall, the data support a role for cAMP in modulating the excitatory action of both FLP17A and 5-HT on C. elegans pharyngeal pumping and furthermore implicate an EGL-30 dependent pathway in the regulation of the response to FLP17A.
Role of cAMP-dependent protein kinase on acute picrotoxin-induced seizures.[Pubmed:16176064]
Neurochem Res. 2005 May;30(5):613-8.
cAMP-dependent protein kinase (PKA) is a major modulator of synaptic transmission likely to be involved in molecular and cellular events leading to epileptogenesis, but little is known about how it affects the onset of acute epileptic seizures. In this study, we determined PKA enzymatic activity in the rat hippocampus during picrotoxin-induced seizures, using H-9 dihydrochloride, a PKA inhibitor, to investigate the in vivo effects of this enzyme on seizures induced by picrotoxin microdialysis in the rat hippocampus. No significant modifications were found in PKA activity during seizures as compared to control rats, but H-9 dihydrochloride microperfusion (100 microM) prevented picrotoxin seizures in 50% of the animals and significantly reduced the mean number of seizures and mean seizure duration. These results suggest that acute picrotoxin-induced seizures occur without an increase in hippocampal PKA activity, but reduced PKA-mediated phosphorylation protects against picrotoxin seizures, probably by increasing the inhibitory potential of GABA(A) receptors. The possibility of other targets for H-9 dihydrochloride, such as PKC, PKG or CAMKII, however, cannot be ruled out.
Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C.[Pubmed:6238627]
Biochemistry. 1984 Oct 9;23(21):5036-41.
Naphthalenesulfonamides such as N-(6-amino-hexyl)-5-chloro-1-naphthalenesulfonamide (W-7) are potent calmodulin (CaM) antagonists and act upon several protein kinases at higher concentration. When the naphthalene ring was replaced by isoquinoline, the derivatives were no longer CaM antagonists but retained the ability to inhibit protein kinases, and some of the derivatives exhibited selective inhibition toward a certain protein kinase. cAMP-dependent, cGMP-dependent, and Ca2+-phospholipid-dependent (protein kinase C) protein kinases were inhibited significantly by addition of 10(-6) M N-[2-(methylamino)ethyl]-5-isoquinoline-sulfonamide (H-8) and 1-(5-isoquinolinylsulfonyl)-2-methylpiperazine (H-7). H-8 was the most active of the inhibitors in this series and inhibited more markedly cyclic nucleotide dependent protein kinases, than other kinases, while the derivative with the sulfonylpiperazine residue (H-7) was the most potent in inhibiting protein kinase C. Apparent Ki values of H-8 were 0.48 and 1.2 microM for cGMP-dependent and cAMP-dependent protein kinases, respectively, and the Ki value of H-7 for protein kinase C was 6 microM. Both the holoenzyme and the catalytic subunit (or fragment), which is active without an enzyme activator, are susceptible to these compounds with a similar concentration dependency, thereby indicating that the inhibitory effect is attributed to the direct interaction of the compound with the active center of the enzyme but not with the enzyme activator. The inhibitions were freely reversible and of the competitive type with respect to ATP and of the noncompetitive type with respect to the phosphate acceptor.(ABSTRACT TRUNCATED AT 250 WORDS)


