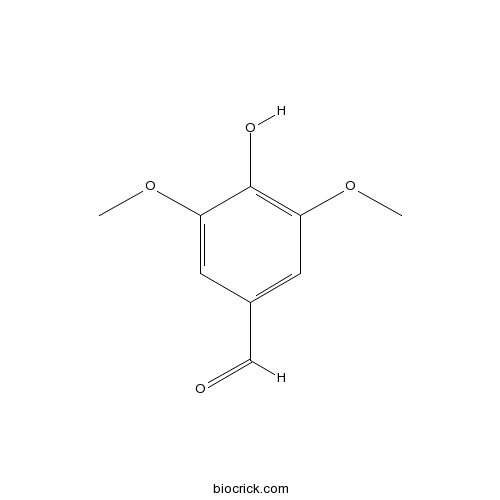
4-Hydroxy-3,5-dimethoxybenzaldehydeCAS# 134-96-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 134-96-3 | SDF | Download SDF |
| PubChem ID | 8655 | Appearance | Powder |
| Formula | C9H10O4 | M.Wt | 182.2 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 4-hydroxy-3,5-dimethoxybenzaldehyde | ||
| SMILES | COC1=CC(=CC(=C1O)OC)C=O | ||
| Standard InChIKey | KCDXJAYRVLXPFO-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 4-Hydroxy-3,5-dimethoxybenzaldehyde is an organic compound that occurs naturally in trace amounts, it possesses powerful antioxidant activity, in minimizing DNA damage, and activating survival signal Akt pathway, and it may be of value in the development of radioprotective compounds. It is used in pharmaceuticals, food, cosmetics, textiles, pulp and paper industries, and even in biological control applications. |
| Targets | DNA-PK | GSK-3 | Akt |
| In vitro | Radioprotection of 4-hydroxy-3,5-dimethoxybenzaldehyde (VND3207) in culture cells is associated with minimizing DNA damage and activating Akt.[Pubmed: 17981442]Eur J Pharm Sci. 2008 Jan;33(1):52-9.Vanillin is a naturally occurring compound and food-flavoring agent with antioxidant and antimutagenic activities.
|
| Structure Identification | Spectrochim Acta A Mol Biomol Spectrosc. 2005 Oct;61(13-14):3087-96.Effects of solvent, pH and beta-cyclodextrin on the photophysical properties of 4-hydroxy-3,5-dimethoxybenzaldehyde: intramolecular charge transfer associated with hydrogen bonding effect.[Pubmed: 16165057]The photophysical properties of 4-Hydroxy-3,5-dimethoxybenzaldehyde (HDMB) in various solvents, pH and in aqueous beta-cyclodextrin (CD) have been investigated. In non-polar solvents, 4-Hydroxy-3,5-dimethoxybenzaldehyde gives only one emission maxima; whereas, in polar solvents it shows a dual luminescence. The increase in Stokes shift with increase in polarity is much more for longer wavelength (LW) than for a shorter wavelength (SW) band. This behaviour indicates the formation of an intramolecular charge transfer (ICT) state through relaxation from the normal excited state. Especially in water, the ICT emission is further red shifted to 430 nm with the normal emission band at 330 nm and the relative fluorescence intensities between 330 nm and 430 nm emission bands are affected by the excitation wavelength. However, this excitation wavelength dependence is not large in aqueous beta-CD solutions. These results suggest that the ICT state in polar solvents/water is stabilized through exciplex formation by the hydrogen-bonding interaction between the carbonyl group and polar solvents/water. The ground and excited state pK(a) values for the neutral-monoanion equilibrium have been measured and discussed. 4-Hydroxy-3,5-dimethoxybenzaldehyde forms a 1:1 inclusion complex with beta-CD. A mechanism is proposed to explain the inclusion process. J Fluoresc. 2014 May;24(3):695-707.Spectral and molecular modeling studies on hydroxybenzaldehydes with native and modified cyclodextrins.[Pubmed: 24310479]The inclusion complexation of 2-hydroxy-3-methoxybenzaldehyde (2HMB), 4-hydroxy-3-methoxybenzaldehyde (4HMB), 3,4-dimethoxybenzaldehyde (DMB) and 4-Hydroxy-3,5-dimethoxybenzaldehyde (HDMB) with α-CD, β-CD, HP-α-CD and HP-β-CD were carried out by UV-Visible, steady-state and time-resolved fluorescence and PM3 methods. All the benzaldehydes shows dual fluorescence in aqueous and CD mediums and 1:1 inclusion complexes were formed with CDs. PM3 geometry optimizations results indicate that the 4-Hydroxy-3,5-dimethoxybenzaldehyde /CD complex is significantly more favorable than the other complexes. The negative enthalpy changes suggest that the inclusion complexation processes are spontaneous. The geometry of the most stable complex shows that methoxy/OH group of HMBs is entrapped in the less polar CD cavities, while the aldehyde group present in the upper part of the CDs cavities. |

4-Hydroxy-3,5-dimethoxybenzaldehyde Dilution Calculator

4-Hydroxy-3,5-dimethoxybenzaldehyde Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.4885 mL | 27.4424 mL | 54.8847 mL | 109.7695 mL | 137.2119 mL |
| 5 mM | 1.0977 mL | 5.4885 mL | 10.9769 mL | 21.9539 mL | 27.4424 mL |
| 10 mM | 0.5488 mL | 2.7442 mL | 5.4885 mL | 10.9769 mL | 13.7212 mL |
| 50 mM | 0.1098 mL | 0.5488 mL | 1.0977 mL | 2.1954 mL | 2.7442 mL |
| 100 mM | 0.0549 mL | 0.2744 mL | 0.5488 mL | 1.0977 mL | 1.3721 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Lobeline Sulphate
Catalog No.:BCC8203
CAS No.:134-64-5
- (-)-Lobeline hydrochloride
Catalog No.:BCC6927
CAS No.:134-63-4
- Azaguanine-8
Catalog No.:BCC4629
CAS No.:134-58-7
- Pelargonidin chloride
Catalog No.:BCN3111
CAS No.:134-04-3
- Sodium ascorbate
Catalog No.:BCC4719
CAS No.:134-03-2
- Peonidin chloride
Catalog No.:BCN3016
CAS No.:134-01-0
- CUDC-907
Catalog No.:BCC2154
CAS No.:1339928-25-4
- Fmoc-Lys(2-Cl-Z)-OH
Catalog No.:BCC3513
CAS No.:133970-31-7
- Aristololactam I
Catalog No.:BCN2456
CAS No.:13395-02-3
- Rimantadine
Catalog No.:BCC4938
CAS No.:13392-28-4
- Kansuiphorin C
Catalog No.:BCN3764
CAS No.:133898-77-8
- ML224
Catalog No.:BCC5596
CAS No.:1338824-21-7
- d-Laserpitin
Catalog No.:BCN3616
CAS No.:134002-17-8
- Phaseollin
Catalog No.:BCN4816
CAS No.:13401-40-6
- Methyl beta-D-fructofuranoside
Catalog No.:BCN6183
CAS No.:13403-14-0
- H-Ala-OtBu.HCl
Catalog No.:BCC3194
CAS No.:13404-22-3
- Selaginellin F
Catalog No.:BCN6420
CAS No.:1340493-24-4
- (RS)-4-Carboxy-3-hydroxyphenylglycine
Catalog No.:BCC6598
CAS No.:134052-66-7
- (R)-4-Carboxyphenylglycine
Catalog No.:BCC6602
CAS No.:134052-68-9
- (S)-4-Carboxyphenylglycine
Catalog No.:BCC6603
CAS No.:134052-73-6
- Ponasterone A
Catalog No.:BCN6184
CAS No.:13408-56-5
- TP-0903
Catalog No.:BCC6462
CAS No.:1341200-45-0
- INCB8761(PF-4136309)
Catalog No.:BCC1649
CAS No.:1341224-83-6
- Fmoc-Tyr(PO3Bzl2)-OH
Catalog No.:BCC3566
CAS No.:134150-51-9
Radioprotection of 4-hydroxy-3,5-dimethoxybenzaldehyde (VND3207) in culture cells is associated with minimizing DNA damage and activating Akt.[Pubmed:17981442]
Eur J Pharm Sci. 2008 Jan;33(1):52-9.
Vanillin is a naturally occurring compound and food-flavoring agent with antioxidant and antimutagenic activities. In present study, we explored the radioprotective effect of a novel vanillin derivative VND3207 (4-Hydroxy-3,5-dimethoxybenzaldehyde). VND3207 has a much higher potential in scavenging hydroxyl radical and superoxide radical than vanillin as indicated in the ESR spin-trapping measurement, and it can effectively protect plasmid DNA against 10-50 Gy gamma-ray induced breaks in vitro at the concentrations as low as 10-20 microM. Using human lymphoblastoid AHH-1 cells and human fibroblastoid HFS cells, we demonstrated that VND3207 at 5-40 microM concentrations significantly attenuated the inhibition of proliferation and occurrence of apoptosis produced by 1-8 Gy gamma-irradiation. In the cultured cells, VND3207 significantly decreased the initial production and residual level of DNA double-strand breaks (DSBs) induced by 2 or 8 Gy irradiation. Treatment of VND3207 enhanced the level of DNA-PKcs protein, a critical component of DNA DSB repair pathway in the cells with or without gamma-irradiation. Consistently, the phosphorylation of Akt protein, a mediator of survival signal, as well as its substrate GSK3beta was concurrently increased by VND3207. Our results suggest that VND3207 has radioprotection effect through its capabilities as a powerful antioxidant, in minimizing DNA damage, and activating survival signal Akt pathway, and it may be of value in the development of radioprotective compounds.
Effects of solvent, pH and beta-cyclodextrin on the photophysical properties of 4-hydroxy-3,5-dimethoxybenzaldehyde: intramolecular charge transfer associated with hydrogen bonding effect.[Pubmed:16165057]
Spectrochim Acta A Mol Biomol Spectrosc. 2005 Oct;61(13-14):3087-96.
The photophysical properties of 4-Hydroxy-3,5-dimethoxybenzaldehyde (HDMB) in various solvents, pH and in aqueous beta-cyclodextrin (CD) have been investigated. In non-polar solvents, HDMB gives only one emission maxima; whereas, in polar solvents it shows a dual luminescence. The increase in Stokes shift with increase in polarity is much more for longer wavelength (LW) than for a shorter wavelength (SW) band. This behaviour indicates the formation of an intramolecular charge transfer (ICT) state through relaxation from the normal excited state. Especially in water, the ICT emission is further red shifted to 430 nm with the normal emission band at 330 nm and the relative fluorescence intensities between 330 nm and 430 nm emission bands are affected by the excitation wavelength. However, this excitation wavelength dependence is not large in aqueous beta-CD solutions. These results suggest that the ICT state in polar solvents/water is stabilized through exciplex formation by the hydrogen-bonding interaction between the carbonyl group and polar solvents/water. The ground and excited state pK(a) values for the neutral-monoanion equilibrium have been measured and discussed. HDMB forms a 1:1 inclusion complex with beta-CD. A mechanism is proposed to explain the inclusion process.
Spectral and molecular modeling studies on hydroxybenzaldehydes with native and modified cyclodextrins.[Pubmed:24310479]
J Fluoresc. 2014 May;24(3):695-707.
The inclusion complexation of 2-hydroxy-3-methoxybenzaldehyde (2HMB), 4-hydroxy-3-methoxybenzaldehyde (4HMB), 3,4-dimethoxybenzaldehyde (DMB) and 4-Hydroxy-3,5-dimethoxybenzaldehyde (HDMB) with alpha-CD, beta-CD, HP-alpha-CD and HP-beta-CD were carried out by UV-Visible, steady-state and time-resolved fluorescence and PM3 methods. All the benzaldehydes shows dual fluorescence in aqueous and CD mediums and 1:1 inclusion complexes were formed with CDs. PM3 geometry optimizations results indicate that the HDMB/CD complex is significantly more favorable than the other complexes. The negative enthalpy changes suggest that the inclusion complexation processes are spontaneous. The geometry of the most stable complex shows that methoxy/OH group of HMBs is entrapped in the less polar CD cavities, while the aldehyde group present in the upper part of the CDs cavities.


