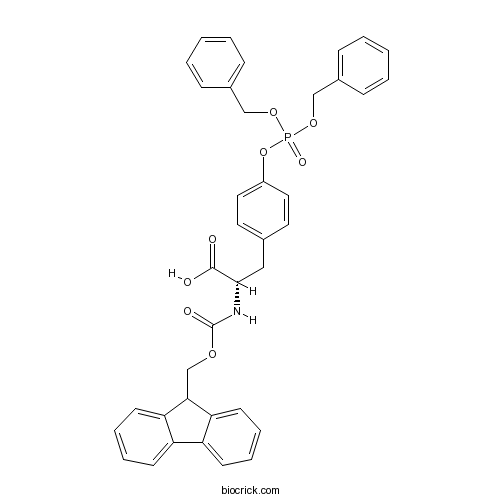
Fmoc-Tyr(PO3Bzl2)-OHCAS# 134150-51-9 |
- PF-4708671
Catalog No.:BCC5031
CAS No.:1255517-76-0
- BIX 02565
Catalog No.:BCC4303
CAS No.:1311367-27-7
- BI-D1870
Catalog No.:BCC5030
CAS No.:501437-28-1
- CMK
Catalog No.:BCC1489
CAS No.:821794-90-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 134150-51-9 | SDF | Download SDF |
| PubChem ID | 15672979 | Appearance | Powder |
| Formula | C38H34NO8P | M.Wt | 663.7 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-3-[4-bis(phenylmethoxy)phosphoryloxyphenyl]-2-(9H-fluoren-9-ylmethoxycarbonylamino)propanoic acid | ||
| SMILES | C1=CC=C(C=C1)COP(=O)(OCC2=CC=CC=C2)OC3=CC=C(C=C3)CC(C(=O)O)NC(=O)OCC4C5=CC=CC=C5C6=CC=CC=C46 | ||
| Standard InChIKey | JSTYRDUOBZALLV-BHVANESWSA-N | ||
| Standard InChI | InChI=1S/C38H34NO8P/c40-37(41)36(39-38(42)44-26-35-33-17-9-7-15-31(33)32-16-8-10-18-34(32)35)23-27-19-21-30(22-20-27)47-48(43,45-24-28-11-3-1-4-12-28)46-25-29-13-5-2-6-14-29/h1-22,35-36H,23-26H2,(H,39,42)(H,40,41)/t36-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Fmoc-Tyr(PO3Bzl2)-OH Dilution Calculator

Fmoc-Tyr(PO3Bzl2)-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5067 mL | 7.5335 mL | 15.067 mL | 30.1341 mL | 37.6676 mL |
| 5 mM | 0.3013 mL | 1.5067 mL | 3.0134 mL | 6.0268 mL | 7.5335 mL |
| 10 mM | 0.1507 mL | 0.7534 mL | 1.5067 mL | 3.0134 mL | 3.7668 mL |
| 50 mM | 0.0301 mL | 0.1507 mL | 0.3013 mL | 0.6027 mL | 0.7534 mL |
| 100 mM | 0.0151 mL | 0.0753 mL | 0.1507 mL | 0.3013 mL | 0.3767 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Fmoc-Tyr(PO3Bzl2)-OH
- INCB8761(PF-4136309)
Catalog No.:BCC1649
CAS No.:1341224-83-6
- TP-0903
Catalog No.:BCC6462
CAS No.:1341200-45-0
- Ponasterone A
Catalog No.:BCN6184
CAS No.:13408-56-5
- (S)-4-Carboxyphenylglycine
Catalog No.:BCC6603
CAS No.:134052-73-6
- (R)-4-Carboxyphenylglycine
Catalog No.:BCC6602
CAS No.:134052-68-9
- (RS)-4-Carboxy-3-hydroxyphenylglycine
Catalog No.:BCC6598
CAS No.:134052-66-7
- Selaginellin F
Catalog No.:BCN6420
CAS No.:1340493-24-4
- H-Ala-OtBu.HCl
Catalog No.:BCC3194
CAS No.:13404-22-3
- Methyl beta-D-fructofuranoside
Catalog No.:BCN6183
CAS No.:13403-14-0
- Phaseollin
Catalog No.:BCN4816
CAS No.:13401-40-6
- d-Laserpitin
Catalog No.:BCN3616
CAS No.:134002-17-8
- 4-Hydroxy-3,5-dimethoxybenzaldehyde
Catalog No.:BCN6186
CAS No.:134-96-3
- Methylcobalamin
Catalog No.:BCC5188
CAS No.:13422-55-4
- RKI-1447
Catalog No.:BCC1903
CAS No.:1342278-01-6
- Daphnelantoxin B
Catalog No.:BCN3228
CAS No.:134273-12-4
- 3,5-Dibromo-4-[3-(dimethylamino)propoxy]cinnamic acid
Catalog No.:BCN1582
CAS No.:134276-56-5
- BIMU 8
Catalog No.:BCC7928
CAS No.:134296-40-5
- Tolcapone
Catalog No.:BCC2334
CAS No.:134308-13-7
- alpha,beta-Methyleneadenosine 5'-triphosphate trisodium salt
Catalog No.:BCC7603
CAS No.:1343364-54-4
- 2-ThioUTP tetrasodium salt
Catalog No.:BCC7625
CAS No.:1343364-70-4
- Epoxymicheliolide
Catalog No.:BCN8275
CAS No.:1343403-10-0
- Ro 0437626
Catalog No.:BCC7276
CAS No.:134362-79-1
- Epoxomicin
Catalog No.:BCC1235
CAS No.:134381-21-8
- Seocalcitol
Catalog No.:BCC1944
CAS No.:134404-52-7
The synthesis and use of pp60src-related peptides and phosphopeptides as substrates for enzymatic phosphorylation studies.[Pubmed:7521748]
Bioorg Med Chem. 1993 Nov;1(5):381-8.
A series of peptides and phosphopeptides corresponding to the auto-phosphorylation site of pp60src, -Asn-Glu-Tyr416-Thr-Ala-, were prepared by either Boc/solution or Fmoc/solid phase peptide synthesis and used as substrates to study their enzymatic phosphorylation by various casein kinases. The Tyr(P)-containing peptide, Asn-Glu-Tyr(P)-Thr-Ala, was prepared by the use of Fmoc-Tyr(PO3Bzl2)-OH in Fmoc/solid phase peptide synthesis followed by acidolytic treatment of the peptide-resin with 5% anisole/CF3CO2H. Both Asn-Glu-Tyr-Thr-Ala and Asn-Glu-Ser(P)-Thr-Ala were prepared by the Boc/solution phase peptide synthesis and employed hydrogenolytic deprotection of the protected peptides. Enzymatic phosphorylation studies established that (A) the Tyr residue acted as an unusual positive determinant for directing phosphorylation to the Thr-residue, (B) the rate of Thr-phosphorylation was markedly facilitated by a change from the Tyr-residue to the Tyr(P)-residue, and (C) a Ser(P)-residue was as effective as the Tyr(P)-residue in facilitating Thr-phosphorylation. A subsequent structure-function study using Asn-Glu-Phe-Thr-Ala, Asn-Glu-Tyr(Me)-Thr-Ala (prepared by Fmoc/solid phase peptide synthesis) and Asn-Glu-Cha-Thr-Ala (prepared by hydrogenation of Asn-Glu-Tyr-Thr-Ala) established that the rate of Thr-phosphorylation was influenced by the extent of hydrophobic-hydrophobic interactions by the aralkyl side-chain group (either aromatic or aliphatic) of the 416-residue with casein kinase-2; the rate of Thr-phosphorylation being decreased by the introduction of methyl or hydroxyl groups at the 4-position of the aromatic group (i.e. Tyr(Me) and Tyr respectively) but enhanced by the introduction of the hydrophilic phosphate group (i.e. as Tyr(P)).


