EpoxomicinProteasome inhibitor CAS# 134381-21-8 |
- MG-115
Catalog No.:BCC1237
CAS No.:133407-86-0
- Clasto-Lactacystin β-lactone
Catalog No.:BCC1224
CAS No.:154226-60-5
- PSI
Catalog No.:BCC1124
CAS No.:158442-41-2
- Salinosporamide A (NPI-0052, Marizomib)
Catalog No.:BCC2094
CAS No.:437742-34-2
- Gliotoxin
Catalog No.:BCN3894
CAS No.:67-99-2
- AM 114
Catalog No.:BCC3589
CAS No.:856849-35-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 134381-21-8 | SDF | Download SDF |
| PubChem ID | 123604 | Appearance | Powder |
| Formula | C28H50N4O7 | M.Wt | 554.7 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BU-4061T | ||
| Solubility | DMSO : 100 mg/mL (180.27 mM; Need ultrasonic) | ||
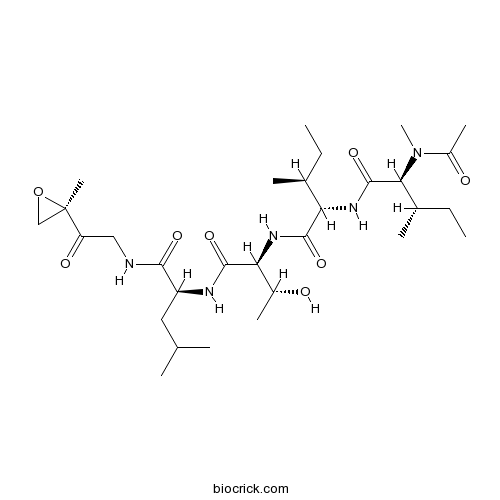
| Chemical Name | (2S,3S)-2-[[(2S,3S)-2-[acetyl(methyl)amino]-3-methylpentanoyl]amino]-N-[(2S,3R)-3-hydroxy-1-[[(2S)-4-methyl-1-[[2-[(2R)-2-methyloxiran-2-yl]-2-oxoethyl]amino]-1-oxopentan-2-yl]amino]-1-oxobutan-2-yl]-3-methylpentanamide | ||
| SMILES | CCC(C)C(C(=O)NC(C(C)O)C(=O)NC(CC(C)C)C(=O)NCC(=O)C1(CO1)C)NC(=O)C(C(C)CC)N(C)C(=O)C | ||
| Standard InChIKey | IODAWNKEPAAFFR-BWEKBLAESA-N | ||
| Standard InChI | InChI=1S/C30H53N5O8/c1-11-17(5)23(33-29(42)25(18(6)12-2)35(10)20(8)37)27(40)34-24(19(7)36)28(41)32-21(13-16(3)4)26(39)31-14-22(38)30(9)15-43-30/h16-19,21,23-25,36H,11-15H2,1-10H3,(H,31,39)(H,32,41)(H,33,42)(H,34,40)/t17-,18-,19+,21-,23-,24-,25-,30+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Epoxomicin is a selective and irreversible inhibitor of 20S proteasome with an IC50 value of 4 nM. | |||||
| Targets | 20S proteasome | |||||
| IC50 | 4 nM | |||||
| Cell experiment:[1] | |
| Cell lines | HEK293T cells |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | Incubated at 0.2 μM or 2 μM epoxomicin for 1 hour |
| Applications | Peptides were degraded by proteasome from cytosolic, mitochondrial, and nuclear proteins. Epoxomicin is a proteasome inhibitor. It decreased the levels of the majority of intracellular peptides, companying with inhibition of the proteasome beta-2 and beta-5 subunits in HEK293T cells. |
| Animal experiment:[2] | |
| Animal models | C57BL6 |
| Dosage form | Epoxomicin (0.58 mg/kg) solubilized in 10% DMSO/PBS were injected i.p. daily for 6 days |
| Application | Epoxomicin reduced inflammation in response to picrylchloride. Epoxomicin potently inhibited the irritant-associated inflammatory response by 95% when ear edema measurements were made 24 hr postchallenge. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: 1. Fricker LD1, Gelman JS, Castro LM et al. Peptidomic analysis of HEK293T cells: effect of the proteasome inhibitor epoxomicin on intracellular peptides. J Proteome Res. 2012 Mar 2;11(3):1981-90. 2. Meng L1, Mohan R, Kwok BH et al. Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity. Proc Natl Acad Sci U S A. 1999 Aug 31;96(18):10403-8. | |

Epoxomicin Dilution Calculator

Epoxomicin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8028 mL | 9.0139 mL | 18.0278 mL | 36.0555 mL | 45.0694 mL |
| 5 mM | 0.3606 mL | 1.8028 mL | 3.6056 mL | 7.2111 mL | 9.0139 mL |
| 10 mM | 0.1803 mL | 0.9014 mL | 1.8028 mL | 3.6056 mL | 4.5069 mL |
| 50 mM | 0.0361 mL | 0.1803 mL | 0.3606 mL | 0.7211 mL | 0.9014 mL |
| 100 mM | 0.018 mL | 0.0901 mL | 0.1803 mL | 0.3606 mL | 0.4507 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
Although it’s a microbial antitumor natural product deemed unfit for clinical development, epoxomicin was turned into YU-101 leading to the discovery of carfilzomin.
Abstract
Epoxomicin at 0.2 or 2 uM decreased levels of the majority of intracellular peptides in HEK294T cells through inhibition of beta-2 and beta-5 subunits of proteasome. However, degradation of proteasome through beta-1 subunit was enabled at a higher concentration of epoxomocon.
Abstract
A five-step pathway of interactions between proteasome and Epoxomicin was described.
Abstract
Epoxomicin is an inhibitor of proteasomal subunits that induces cell death through accumulation of ubiquinated proteins. Epoxomicin exhibited potent in vitro and in vivo inhibition against babesiosis alone or in combination with diminazene aceturate.
Abstract
Epoxomicin, a proteasome inhibitor, not only exhibited potent antimalarial activity killing malaria parasites without affecting normal cells but also inhibited oocyst production in the mosquito midgut.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Epoxomicin was originally isolated from the culture medium of an Actinomycetes strain based on its in vivo antitumor activity against murine B16 melanoma. Epoxomicin is a naturally occurring selective proteasome inhibitor with anti-inflammatory activity. [1] Epoxomicin primarily inhibits the activity of CTRL (chymotrypsin-like proteasome).
The novel α-epoxy ketone moiety of Epoxomicin forms covalent bonds with residues in particular catalytic subunits of the enzyme, disabling activity. The trypsin-like and peptidyl-glutamyl peptide hydrolyzing behaviors of the proteasome were both inhibited by Epoxomicin as well (at 100 and 1,000-fold slower rates, respectively). The ubiquitin-proteasome pathway heavily regulates bone formation, and Epoxomicin was shown to increase both bone volume and bone formation rates in rodents.
Another study demonstrates that exposure to Epoxomicin and other proteasome inhibitors leads to dopaminergic cell death, producing a model of Parkinson's disease in vivo. Epoxomicin is an inhibitor of 20S Proteasome. [2]
References:
1. Meng, L; Mohan, R; Kwok, BH; Elofsson, M; Sin, N; Crews, CM (1999). "Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity". PNAS 96 (18): 10403–10408.
2. Epoxomicin, Santa Cruz Biotechnology.
- Ro 0437626
Catalog No.:BCC7276
CAS No.:134362-79-1
- Epoxymicheliolide
Catalog No.:BCN8275
CAS No.:1343403-10-0
- 2-ThioUTP tetrasodium salt
Catalog No.:BCC7625
CAS No.:1343364-70-4
- alpha,beta-Methyleneadenosine 5'-triphosphate trisodium salt
Catalog No.:BCC7603
CAS No.:1343364-54-4
- Tolcapone
Catalog No.:BCC2334
CAS No.:134308-13-7
- BIMU 8
Catalog No.:BCC7928
CAS No.:134296-40-5
- 3,5-Dibromo-4-[3-(dimethylamino)propoxy]cinnamic acid
Catalog No.:BCN1582
CAS No.:134276-56-5
- Daphnelantoxin B
Catalog No.:BCN3228
CAS No.:134273-12-4
- RKI-1447
Catalog No.:BCC1903
CAS No.:1342278-01-6
- Methylcobalamin
Catalog No.:BCC5188
CAS No.:13422-55-4
- Fmoc-Tyr(PO3Bzl2)-OH
Catalog No.:BCC3566
CAS No.:134150-51-9
- INCB8761(PF-4136309)
Catalog No.:BCC1649
CAS No.:1341224-83-6
- Seocalcitol
Catalog No.:BCC1944
CAS No.:134404-52-7
- Dehydroandrographolide
Catalog No.:BCN1260
CAS No.:134418-28-3
- CA 074
Catalog No.:BCC1141
CAS No.:134448-10-5
- Discodermide
Catalog No.:BCN1834
CAS No.:134458-00-7
- BW-B 70C
Catalog No.:BCC7013
CAS No.:134470-38-5
- Richenoic acid
Catalog No.:BCN6185
CAS No.:134476-74-7
- Trimethylvinylammonium(1+)
Catalog No.:BCN1820
CAS No.:13448-18-5
- 1-Cinnamoyl-3-hydroxypyrrolidine
Catalog No.:BCN6497
CAS No.:1344876-77-2
- Gardenoin J
Catalog No.:BCN7666
CAS No.:1345109-46-7
- U 90042
Catalog No.:BCC7465
CAS No.:134516-99-7
- Atorvastatin Calcium
Catalog No.:BCC2319
CAS No.:134523-03-8
- AM095
Catalog No.:BCC1351
CAS No.:1345614-59-6
Regulation of ubiquitin-proteasome and autophagy pathways after acute LPS and epoxomicin administration in mice.[Pubmed:24885455]
BMC Musculoskelet Disord. 2014 May 22;15:166.
BACKGROUND: The ubiquitin-proteasome pathway (UPP) is a major protein degradation pathway that is activated during sepsis and has been proposed as a therapeutic target for preventing skeletal muscle loss due to cachexia. Although several studies have investigated the modulation of proteasome activity in response to LPS administration, none have characterized the overall UPP response to LPS administration in the fate of proteasome inhibition. METHODS: Here, we determined the modulation pattern of the main key components of the UPP in the gastrocnemius (GAS) of mice during the acute phase of lipopolysaccharide (LPS)-mediated endotoxemia (7.5 mg/kg - 8 h) by measuring all three beta1, beta2 and beta5 activites of the 20S and 26S proteasomes, the levels of steady state polyubiquitinated proteins, mRNA levels of muscle ligases, as well as signaling pathways regulating the UPP. Another goal was to assess the effects of administration of a specific proteasome inhibitor (Epoxomicin, 0.5 mg/kg) on UPP response to sepsis. RESULTS: The acute phase of LPS-induced endotoxemia lowered GAS/body weight ratio and increased MuRF1 and MAFbx mRNA concomitantly to an activation of the pathways known to regulate their expression. Unexpectedly, we observed a decrease in all 20S and 26S proteasome activities measured in GAS, which might be related to oxidative stress, as oxidized proteins (carbonyl levels) increase with LPS. While significantly inhibiting 20S and 26S proteasome beta5 activities in heart and liver, Epoxomicin did not lower proteasome activity in GAS. However, the increase in mRNA expression of the muscle ligases MuRF1 and MAFbx were partially rescued without affecting the other investigated signaling pathways. LPS also strongly activated autophagy, which could explain the observed GAS atrophy with LPS-induced reduction of proteasome activity. CONCLUSIONS: Our results highlight an opposite regulation of UPP in the early hours of LPS-induced muscle atrophy by showing reduced proteasome activities and increased mRNA expression of muscle specific ligases. Furthermore, our data do not support any preventive effect of Epoxomicin in muscle atrophy due to acute cachexia since proteasome activities are not further repressed.
Epoxomicin Sensitizes Resistant Osteosarcoma Cells to TRAIL Induced Apoptosis.[Pubmed:25666501]
Anticancer Agents Med Chem. 2015;15(4):527-33.
Osteosarcoma (OS) is the second most common primary malign bone neoplasm after multiple myeloma. Despite systemic chemotherapy, OS may give rise to local recurrences and metastases. Resistance to chemotherapy is not rare and is likely to occur in a high number of patients. Novel therapeutic approaches are required in order to efficiently treat osteosarcoma. Tumor necrosis factor (TNF)-related apoptosis inducing ligand (TRAIL) and proteasome inhibitors (Epoxomicin, MG132, bortezomib) represent new promising approaches in cancer treatment. The aim of our study is to elucidate the effects of Epoxomicin alone or in combination with TRAIL in two TRAIL-resistant OS cell lines, Saos-2 and MG-63 namely. We determined the cytotoxic effects of Epoxomicin and/or TRAIL on these two types of OS cells using dimethylthiazolyl 2,5 diphenyltetrazolium bromide (MTT) test and measured apoptosis markers such as pro-apoptotic Bax levels and caspase-3, -8, -9 activities. We used TUNEL assay to demonstrate apoptosis. We investigated dose and time dependent survival rates of OS cells and determined LD50 doses of Epoxomicin and TRAIL on OS cell viability after 24, 48, and 72 hour incubations. Concurrent incubation with TRAIL and Epoxomicin for 24 hour significantly increased caspase-3, caspase-8, caspase-9 activities and Bax protein levels. Our study demonstrated that the combination of TRAIL with Epoxomicin enhances apoptosis, and overcomes TRAIL resistance, denoting promising results for OS therapy in the future.
Epoxomicin and Eponemycin Biosynthesis Involves gem-Dimethylation and an Acyl-CoA Dehydrogenase-Like Enzyme.[Pubmed:26789439]
Chembiochem. 2016 May 3;17(9):792-8.
The alpha',beta'-epoxyketone moiety of proteasome inhibitors confers high binding specificity to the N-terminal threonine in catalytic proteasome beta-subunits. We recently identified the Epoxomicin and eponemycin biosynthetic gene clusters and have now conducted isotope-enriched precursor feeding studies and comprehensive gene deletion experiments to shed further light on their biosynthetic pathways. Leucine and two methyl groups from S-adenosylmethionine were readily incorporated into the epoxyketone warhead, suggesting decarboxylation of the thioester intermediate. Formation of the alpha',beta'-epoxyketone is likely mediated by conserved acyl-CoA dehydrogenase-like enzymes, as indicated by complete loss of Epoxomicin and eponemycin production in the respective knockout mutants. Our results clarify crucial questions in the formation of epoxyketone compounds and lay the foundation for in vitro biochemical studies on the biosynthesis of this pharmaceutically important class of proteasome inhibitors.
From epoxomicin to carfilzomib: chemistry, biology, and medical outcomes.[Pubmed:23575525]
Nat Prod Rep. 2013 May;30(5):600-4.
The initial enthusiasm following the discovery of a pharmacologically active natural product is often fleeting due to the poor prospects for its ultimate clinical application. Despite this, the ever-changing landscape of modern biology has a constant need for molecular probes that can aid in our understanding of biological processes. After its initial discovery by Bristol-Myers Squibb as a microbial anti-tumor natural product, Epoxomicin was deemed unfit for development due to its peptide structure and potentially labile epoxyketone pharmacophore. Despite its drawbacks, Epoxomicin's pharmacophore was found to provide unprecedented selectivity for the proteasome. Epoxomicin also served as a scaffold for the generation of a synthetic tetrapeptide epoxyketone with improved activity, YU-101, which became the parent lead compound of carfilzomib (Kyprolis), the recently approved therapeutic agent for multiple myeloma. In this era of rational drug design and high-throughput screening, the prospects for turning an active natural product into an approved therapy are often slim. However, by understanding the journey that began with the discovery of Epoxomicin and ended with the successful use of carfilzomib in the clinic, we may find new insights into the keys for success in natural product-based drug discovery.


