SB 200646 hydrochloride5-HT2C/2B antagonist CAS# 143797-62-0 |
- Trelagliptin succinate
Catalog No.:BCC2015
CAS No.:1029877-94-8
- Alogliptin Benzoate
Catalog No.:BCC1341
CAS No.:850649-62-6
- Trelagliptin
Catalog No.:BCC2014
CAS No.:865759-25-7
- Teneligliptin hydrobromide
Catalog No.:BCC1992
CAS No.:906093-29-6
- Glimepiride
Catalog No.:BCC2109
CAS No.:93479-97-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 143797-62-0 | SDF | Download SDF |
| PubChem ID | 5311422 | Appearance | Powder |
| Formula | C15H15ClN4O | M.Wt | 302.76 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
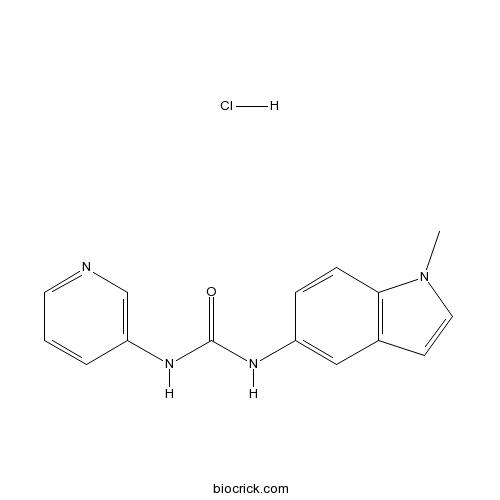
| Chemical Name | 1-(1-methylindol-5-yl)-3-pyridin-3-ylurea;hydrochloride | ||
| SMILES | CN1C=CC2=C1C=CC(=C2)NC(=O)NC3=CN=CC=C3.Cl | ||
| Standard InChIKey | IGRYPUQJEDJLHC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H14N4O.ClH/c1-19-8-6-11-9-12(4-5-14(11)19)17-15(20)18-13-3-2-7-16-10-13;/h2-10H,1H3,(H2,17,18,20);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 5-HT2C/2B receptor antagonist, selective over 5-HT1A. Affinities are 7.4 (pA2), 6.9 (pKi) and 5.2 (pKi) for 5-HT2B, 2C and 2A respectively. Orally active in vivo. |

SB 200646 hydrochloride Dilution Calculator

SB 200646 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3029 mL | 16.5147 mL | 33.0295 mL | 66.0589 mL | 82.5737 mL |
| 5 mM | 0.6606 mL | 3.3029 mL | 6.6059 mL | 13.2118 mL | 16.5147 mL |
| 10 mM | 0.3303 mL | 1.6515 mL | 3.3029 mL | 6.6059 mL | 8.2574 mL |
| 50 mM | 0.0661 mL | 0.3303 mL | 0.6606 mL | 1.3212 mL | 1.6515 mL |
| 100 mM | 0.033 mL | 0.1651 mL | 0.3303 mL | 0.6606 mL | 0.8257 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3-O-Coumaroylasiatic acid
Catalog No.:BCN7132
CAS No.:143773-52-8
- (RS)-Abscisic acid
Catalog No.:BCN8353
CAS No.:14375-45-2
- PACAP 6-38
Catalog No.:BCC7611
CAS No.:143748-18-9
- Pyrazine-2-carbaldehyde
Catalog No.:BCN2565
CAS No.:5780-66-5
- Kaempferol 3,4,7-triacetate
Catalog No.:BCN6242
CAS No.:143724-69-0
- Soyasaponin Bd
Catalog No.:BCN2465
CAS No.:135272-91-2
- CE3F4
Catalog No.:BCC5605
CAS No.:143703-25-7
- GYKI 53655 hydrochloride
Catalog No.:BCC7407
CAS No.:143692-48-2
- Fmoc-D-Ile-OPfp
Catalog No.:BCC3507
CAS No.:143688-83-9
- G-Protein antagonist peptide
Catalog No.:BCC7206
CAS No.:143675-79-0
- Elacridar
Catalog No.:BCC1546
CAS No.:143664-11-3
- Diacetoxy-6-gingerdiol
Catalog No.:BCN3339
CAS No.:143615-75-2
- 13-Epimanool
Catalog No.:BCN4862
CAS No.:1438-62-6
- 22-Dehydroclerosterol glucoside
Catalog No.:BCN6243
CAS No.:143815-99-0
- Fmoc-Trp(Boc)-OH
Catalog No.:BCC3558
CAS No.:143824-78-6
- 2,24-Dihydroxyursolic acid
Catalog No.:BCN6244
CAS No.:143839-02-5
- CC-401 hydrochloride
Catalog No.:BCC1458
CAS No.:1438391-30-0
- Elacridar hydrochloride
Catalog No.:BCC1547
CAS No.:143851-98-3
- M40
Catalog No.:BCC7686
CAS No.:143896-17-7
- CB-839
Catalog No.:BCC5493
CAS No.:1439399-58-2
- Jaceidin triacetate
Catalog No.:BCN6245
CAS No.:14397-69-4
- CTX0294885
Catalog No.:BCC6396
CAS No.:1439934-41-4
- Sodium barbital
Catalog No.:BCN2160
CAS No.:144-02-5
- Sodium bicarbonate
Catalog No.:BCC7584
CAS No.:144-55-8
SB-616234-A (1-[6-(cis-3,5-dimethylpiperazin-1-yl)-2,3-dihydro-5-methoxyindol-1-yl]-1-[2'meth yl-4'-(5-methyl-1,2,3-oxadiazol-3-yl)biphenyl-4-yl]methanone hydrochloride): a novel, potent and selective 5-HT1B receptor antagonist.[Pubmed:16546225]
Neuropharmacology. 2006 Jun;50(8):984-90.
SB-616234-A possesses high affinity for human 5-HT1B receptors stably expressed in Chinese hamster ovary (CHO) cells (pKi 8.3+/-0.2), and is over 100-fold selective for a range of molecular targets except h5-HT1) receptors (pKi 6.6+/-0.1). Similarly, affinity (pKi) for rat and guinea pig striatal 5-HT1B receptors is 9.2+/-0.1. In [35S]-GTPgammaS binding studies in the human recombinant cell line, SB-616234-A acted as a high affinity antagonist with a pA2 value of 8.6+/-0.2 whilst providing no evidence of agonist activity in this system. In [35S]-GTPgammaS binding studies in rat striatal membranes, SB-616234-A acted as a high affinity antagonist with an apparent pKB of 8.4+/-0.5, again whilst providing no evidence of agonist activity in this system. SB-616234-A (1 microM) potentiated electrically stimulated [3H]-5-HT release from guinea pig and rat cortical slices (S2/S1) ratios of 1.8 and 1.6, respectively). SB-616234-A (0.3-30 mg kg(-1) p.o.) caused a dose-dependent inhibition of ex vivo [3H]-GR125743 binding to rat striatal 5-HT1B receptors with an ED50 of 2.83+/-0.39 mg kg(-1) p.o. Taken together these data suggest that SB-616234-A is a potent and selective 5-HT(1B) autoreceptor antagonist that occupies central 5-HT1B receptors in vivo following oral administration.
Clinical tolerability of ibopamine hydrochloride (SB 7505).[Pubmed:7250173]
Eur J Clin Pharmacol. 1981;19(6):409-11.
The clinical tolerance of ibopamine hydrochloride (Sb 7505) was investigated in 12 volunteers. The drug was administered on alternate days (2nd, 4th, 6th, 8th, 10th, 12th), starting at 100 mg and increasing by 50 mg each time to reach 350 mg on the 12th day. On the other days (1st, 3rd, 5th, 7th, 9th, 11th and 13th) a placebo was given. Diuresis increased progressively with the dose, reaching a maximum increase of 88% after the last dose, and showing a small residual effect on each subsequent placebo day. Body weight showed a marginal change and decreased by 2% in the last two days of treatment. Heart rate, systolic, diastolic and mean blood pressure showed only marginal fluctuations of about 7% around the mean values, which were of little statistical and of no clinical significance. Haematological and biochemical parameters were not affected. No side effect was noticed. The dose of 350 mg may probably be increased without leading to side effects.
Characterisation of the selective 5-HT1B receptor antagonist SB-616234-A (1-[6-(cis-3,5-dimethylpiperazin-1-yl)-2,3-dihydro-5-methoxyindol-1-yl]-1-[2'-met hyl-4'-(5-methyl-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]methanone hydrochloride): in vivo neurochemical and behavioural evidence of anxiolytic/antidepressant activity.[Pubmed:16581092]
Neuropharmacology. 2006 Jun;50(8):975-83.
The 5-HT1B receptor has attracted significant interest as a potential target for the development of therapeutics for the treatment of affective disorders such as anxiety and depression. Here we present the in vivo characterisation of a novel, selective and orally bioavailable 5-HT1B receptor antagonist, SB-616234-A (1-[6-(cis-3,5-dimethylpiperazin-1-yl)-2,3-dihydro-5-methoxyindol-1-yl]-1-[2'-met hyl-4'-(5-methyl-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]methanone hydrochloride). SB-616234-A reversed the 5-HT1/7 receptor agonist, SKF-99101H-induced hypothermia in guinea pigs in a dose related manner with an ED50 of 2.4 mg/kg p.o. Using in vivo microdialysis in freely moving guinea pigs, SB-616234-A (3-30 mg/kg p.o.) caused a dose-related increase in extracellular 5-HT in the dentate gyrus. Evaluation of antidepressant- and anxiolytic-like effects of this 5-HT1B receptor antagonist was performed in a variety of models and species. SB-616234-A produced a decrease in immobility time in the mouse forced swim test; an effect suggestive of antidepressant activity. Furthermore, SB-616234-A produced dose-related anxiolytic effects in both rat and guinea pig maternal separation-induced vocalisation models with an ED50 of 1.0 and 3.3 mg/kg i.p., respectively (vs fluoxetine treatment ED50 = 2.2 mg/kg i.p. in both species). Also a significant reduction in posturing behaviours was observed in the human threat test in marmosets; an effect indicative of anxiolytic activity. In summary, SB-616234-A is a novel, potent and orally bioavailable 5-HT1B receptor antagonist which exhibits a neurochemical and behavioural profile that is consistent with both anxiolytic- and antidepressant-like activity in a variety of species. Taken together these data suggest that SB-616234-A may have therapeutic efficacy in the treatment of affective disorders.
Evidence for a selective role of the delta-opioid agonist [8R-(4bS*,8aalpha,8abeta, 12bbeta)]7,10-Dimethyl-1-methoxy-11-(2-methylpropyl)oxycarbonyl 5,6,7,8,12,12b-hexahydro-(9H)-4,8-methanobenzofuro[3,2-e]pyrrolo[2,3-g]isoquinoli ne hydrochloride (SB-235863) in blocking hyperalgesia associated with inflammatory and neuropathic pain responses.[Pubmed:14551288]
J Pharmacol Exp Ther. 2003 Dec;307(3):1079-89.
The specific involvement of the delta-opioid receptor in the control of nociception was explored by investigating the pharmacological activity in vivo of a selective, orally active, and centrally penetrant delta-opioid agonist. [8R-(4bS*,8aalpha,8abeta,12bbeta)]7,10-dimethyl-1-methoxy-11-(2-methylpropyl)oxyc arbonyl 5,6,7,8,12,12b-hexahydro-(9H)-4,8-methanobenzofuro[3,2-e]pyrrolo[2,3-g]isoquinoli ne hydrochloride (SB-235863) is a new pyrrolomorphinan with high affinity (Ki = 4.81 +/- 0.39 nM) for the delta-opioid receptor, full agonist activity, and binding selectivity versus the mu- and kappa-opioid receptors of 189-fold and 52-fold, respectively. Perorally administered SB-236863 was inactive in the rat tail-flick and hot-plate tests of acute pain response, but potently reversed thermal hyperalgesia in rats resulting from a carrageenan-induced inflammatory response. This activity could be blocked by the delta-opioid antagonist naltrindole (3 mg/kg s.c.), but selective mu- and kappa-opioid antagonists were ineffective. Naltrindole (1 microg i.c.v.) also blocked the activity of 10 mg/kg (p.o.) SB-235863, showing that the compound activates delta-opioid receptor sites in the central nervous system. SB-235863 was additionally effective at reversing chronic hyperalgesia in the Seltzer rat model of partial sciatic nerve ligation after peroral administration. These data show that the delta-opioid receptor plays a selective role in regulating evoked and lasting changes in nociceptive pain signaling. Classical side effects of mu- and kappa-opioid receptor activation (slowing of gastrointestinal transit and motor incoordination, respectively) were not observed after administration of 70 mg/kg (p.o.) SB-235863, nor was evoked seizure activity affected. These results suggest a selective and limited role of delta-opioid receptors in the modulation of nociception.
Effect of SB 200646A, a 5-HT2C/5-HT2B receptor antagonist, in two conflict models of anxiety.[Pubmed:7617805]
Psychopharmacology (Berl). 1995 Mar;118(2):178-82.
SB 200646A is the first selective 5-HT2C/5-HT2B receptor antagonist and has previously been observed to have anxiolytic-like properties in the rat social interaction test. In the present study the effects of the compound in two conflict models of anxiety, the rat Geller-Seifter and marmoset conflict test, were examined. In the rat Geller-Seifter test, suppressed responding was increased by all doses of SB 200646A between 5 and 40 mg/kg PO when given 1 h pretest. Unsuppressed responding was slightly increased only at 10 mg/kg PO. Suppressed responding was also increased by the benzodiazepine anxiolytic, chlordiazepoxide, at 1, 2.5 and 5 mg/kg PO 1 h pretest. Unsuppressed responding was modestly increased by chlordiazepoxide only at 5 mg/kg PO. In the marmoset conflict test marmosets were trained to lever press for a palatable food reward. Lever pressing was subsequently suppressed by air puffs. In this procedure suppressed responding was increased by both the benzodiazepine anxiolytic diazepam at 2 and 5 mg/kg PO and SB 200646A after 10 and 20 mg/kg PO. Both treatments caused small increases in unsuppressed responding at 2 and 20 mg/kg PO respectively. Taken together with the previous effects of SB 200646A in the rat social interaction test, this is compelling evidence that 5-HT2C/2B receptor antagonists may possess anxiolytic properties.
In vivo properties of SB 200646A, a 5-HT2C/2B receptor antagonist.[Pubmed:7912626]
Br J Pharmacol. 1994 Mar;111(3):797-802.
1. SB 200646A, N-(1-methyl-5-indolyl)-N'-(3-pyridyl) urea hydrochloride, the first reported selective 5-HT2C/2B over 5-HT2A receptor antagonist, (pK1 rat 5-HT2C receptor 6.9, pA2 rat 5-HT2B receptor 7.5, pK1 rat 5-HT2A receptor 5.2) dose-dependently blocked a putative rat model of 5-HT2C receptor activation; 1-(3-chlorophenyl)piperazine (mCPP, 5 mg kg-1, i.p. 20 min pretest)-induced hypolocomotion (estimated ID50 19.2 mg kg-1, p.o.). 2. SB 200646A also blocked another putative in vivo model of 5-HT2C receptor function; mCPP (5 mg kg-1, i.p. 20 min pretest)-induced hypophagia in 23 h food-deprived rats (estimated ID50 18.3 mg kg-1, p.o.). 3. SB 200646A did not antagonize 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI)-induced head shakes in rats at doses up to 200 mg kg-1, p.o., an effect thought to be mediated by 5-HT2A receptors for which SB 200646A has its next highest affinity (50 fold less) after the 5-HT2C and 5-HT2B sites. 4. SB 200646A (20, 40 mg kg-1, p.o., 1 h pretest) also reversed mCPP (0.5 mg kg-1, i.p., 30 min pretest)-induced anxiety in the social interaction test, under low light familiar conditions. 5. When given alone, under high light unfamiliar conditions, SB 200646A (2-40 mg kg-1, p.o.) increased active social interaction without affecting locomotor activity in the rat social interaction test. This is consistent with an anxiolytic action of SB 200646A. 6. These results indicate that SB 200646A has in vivo efficacy and that 5-HT2C or 5-HT2B receptors are indeed likely to mediate mCPP-induced hypolocomotion, hypophagia and anxiogenesis. They also suggest that 5-HT2C,2B receptor blockade induces anxiolysis.


