Soyasaponin BdCAS# 135272-91-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 135272-91-2 | SDF | Download SDF |
| PubChem ID | 101672146 | Appearance | Powder |
| Formula | C48H76O19 | M.Wt | 957.1 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
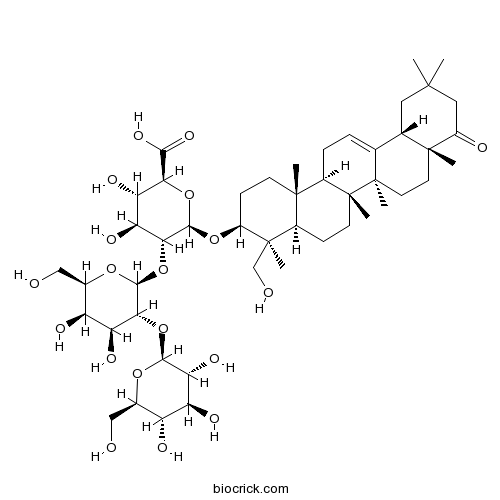
| Chemical Name | (2S,3S,4S,5R,6R)-6-[[(3S,4S,4aR,6aR,6bS,8aR,12aS,14aR,14bR)-4-(hydroxymethyl)-4,6a,6b,8a,11,11,14b-heptamethyl-9-oxo-2,3,4a,5,6,7,8,10,12,12a,14,14a-dodecahydro-1H-picen-3-yl]oxy]-5-[(2S,3R,4S,5R,6R)-4,5-dihydroxy-6-(hydroxymethyl)-3-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyoxan-2-yl]oxy-3,4-dihydroxyoxane-2-carboxylic acid | ||
| SMILES | CC1(CC2C3=CCC4C5(CCC(C(C5CCC4(C3(CCC2(C(=O)C1)C)C)C)(C)CO)OC6C(C(C(C(O6)C(=O)O)O)O)OC7C(C(C(C(O7)CO)O)O)OC8C(C(C(C(O8)CO)O)O)O)C)C | ||
| Standard InChIKey | JTXVTHCLTOUSSL-VSDZMWCXSA-N | ||
| Standard InChI | InChI=1S/C48H76O19/c1-43(2)16-22-21-8-9-26-45(4)12-11-28(46(5,20-51)25(45)10-13-48(26,7)47(21,6)15-14-44(22,3)27(52)17-43)64-42-38(34(58)33(57)36(65-42)39(60)61)67-41-37(32(56)30(54)24(19-50)63-41)66-40-35(59)31(55)29(53)23(18-49)62-40/h8,22-26,28-38,40-42,49-51,53-59H,9-20H2,1-7H3,(H,60,61)/t22-,23+,24+,25+,26+,28-,29+,30-,31-,32-,33-,34-,35+,36-,37+,38+,40-,41-,42+,44+,45-,46+,47+,48+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cinnamic aldehyde, a COX-2 inhibitor, exhibits cardioprotective, antidepressant-like, anti-leukemia, anti-oxidative and anti-inflammatory properties. Its supplementation can improve glucose and lipid homeostasis in diabetic animals. |
| Targets | COX | PGE | TNF-α | IL Receptor | NO | ATPase | SOD |
| In vitro | Mechanism of cinnamic aldehyde-inducing apoptosis of chronic myeloid leukemic cells in vitro.[Pubmed: 21729535]Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2011 Jun;19(3):617-20.The aim of this study was to investigate the apoptosis-inducing effect of Cinnamic aldehyde (CA) on chronic myeloid leukemic (CML) cells and its mechanism. Potent nematicidal activity of phthalaldehyde, salicylaldehyde, and cinnamic aldehyde against Meloidogyne incognita.[Pubmed: 23379671]J Agric Food Chem. 2013 Feb 27;61(8):1794-803.
|
| In vivo | Cinnamic aldehyde treatment alleviates chronic unexpected stress-induced depressive-like behaviors via targeting cyclooxygenase-2 in mid-aged rats.[Pubmed: 25556926]J Ethnopharmacol. 2015 Mar 13;162:97-103. COX-2 has been considered as a potent molecular target for prevention and therapy of depression. However, a recent study showed that COX-2 inhibitor does not improve depressive symptoms in persons aged 70 and over. Therefore, whether treatments targeting COX-2 have a clinical efficacy in depression, especially elderly individuals, remains unclear. Cinnamic aldehyde is a major constituent of Cinnamomum cassia, which has exhibited excellent anti-inflammatory activities as a COX-2 inhibitor. To investigate the potential antidepressant effect of Cinnamic aldehyde in mid-aged rats. |
| Animal Research | Protective effects of cinnamic acid and cinnamic aldehyde on isoproterenol-induced acute myocardial ischemia in rats.[Pubmed: 24001892]J Ethnopharmacol. 2013 Oct 28;150(1):125-30.Cinnamomum cassia is a well-known traditional Chinese herb that is widely used for the treatment of ischemic heart disease (IHD). It has favorable effects, but its mechanism is not clear. To investigate the effects of Cinnamic aldehyde (CA) and cinnamic acid (CD) isolated from Cinnamomum cassia against myocardial ischemia produced in rats by isoproterenol (ISO). |

Soyasaponin Bd Dilution Calculator

Soyasaponin Bd Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.0448 mL | 5.2241 mL | 10.4482 mL | 20.8965 mL | 26.1206 mL |
| 5 mM | 0.209 mL | 1.0448 mL | 2.0896 mL | 4.1793 mL | 5.2241 mL |
| 10 mM | 0.1045 mL | 0.5224 mL | 1.0448 mL | 2.0896 mL | 2.6121 mL |
| 50 mM | 0.0209 mL | 0.1045 mL | 0.209 mL | 0.4179 mL | 0.5224 mL |
| 100 mM | 0.0104 mL | 0.0522 mL | 0.1045 mL | 0.209 mL | 0.2612 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- CE3F4
Catalog No.:BCC5605
CAS No.:143703-25-7
- GYKI 53655 hydrochloride
Catalog No.:BCC7407
CAS No.:143692-48-2
- Fmoc-D-Ile-OPfp
Catalog No.:BCC3507
CAS No.:143688-83-9
- G-Protein antagonist peptide
Catalog No.:BCC7206
CAS No.:143675-79-0
- Elacridar
Catalog No.:BCC1546
CAS No.:143664-11-3
- Diacetoxy-6-gingerdiol
Catalog No.:BCN3339
CAS No.:143615-75-2
- (2R,3S)-3-Phenylisoserine ethyl ester
Catalog No.:BCC8388
CAS No.:143615-00-3
- 3-(4-Chlorobutyl)indole-5-carbonitrile
Catalog No.:BCC8589
CAS No.:143612-79-7
- Curculigoside B
Catalog No.:BCN7939
CAS No.:143601-09-6
- 5-Hydroxy-2-(4-methoxyphenyl)-8,8-dimethyl-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxypyrano[2,3-h]chromen-4-one
Catalog No.:BCC8807
CAS No.:143601-07-4
- Cyclo(Leu-Leu)
Catalog No.:BCN2433
CAS No.:1436-27-7
- A 419259 trihydrochloride
Catalog No.:BCC4308
CAS No.:1435934-25-0
- Kaempferol 3,4,7-triacetate
Catalog No.:BCN6242
CAS No.:143724-69-0
- Pyrazine-2-carbaldehyde
Catalog No.:BCN2565
CAS No.:5780-66-5
- PACAP 6-38
Catalog No.:BCC7611
CAS No.:143748-18-9
- (RS)-Abscisic acid
Catalog No.:BCN8353
CAS No.:14375-45-2
- 3-O-Coumaroylasiatic acid
Catalog No.:BCN7132
CAS No.:143773-52-8
- SB 200646 hydrochloride
Catalog No.:BCC5751
CAS No.:143797-62-0
- 13-Epimanool
Catalog No.:BCN4862
CAS No.:1438-62-6
- 22-Dehydroclerosterol glucoside
Catalog No.:BCN6243
CAS No.:143815-99-0
- Fmoc-Trp(Boc)-OH
Catalog No.:BCC3558
CAS No.:143824-78-6
- 2,24-Dihydroxyursolic acid
Catalog No.:BCN6244
CAS No.:143839-02-5
- CC-401 hydrochloride
Catalog No.:BCC1458
CAS No.:1438391-30-0
- Elacridar hydrochloride
Catalog No.:BCC1547
CAS No.:143851-98-3
Soyasaponins from Zolfino bean as aldose reductase differential inhibitors.[Pubmed:30734590]
J Enzyme Inhib Med Chem. 2019 Dec;34(1):350-360.
Seven triterpenoid saponins were identified in methanolic extracts of seeds of the Zolfino bean landrace (Phaseolus vulgaris L.) by HPLC fractionation, revealing their ability to inhibit highly purified human recombinant aldose reductase (hAKR1B1). Six of these compounds were associated by MS analysis with the following saponins already reported in different Phaseolus vulgaris varieties: soyasaponin Ba (V), soyasaponin Bb, Soyasaponin Bd (sandosaponin A), soyasaponin alphag, 3-O-[R-l-rhamnopyranosyl(1 --> 2)-alpha-d-glucopyranosyl(1 --> 2)-alpha-d-glucuronopyranosyl]olean-12-en-22-oxo-3alpha,-24-diol, and soyasaponin betag. The inhibitory activity of the collected fractions containing the above compounds was tested for hAKR1B1-dependent reduction of both l-idose and 4-hydroxynonenal, revealing that some are able to differentially inhibit the enzyme. The present work also highlights the difficulties in the search for aldose reductase differential inhibitors (ARDIs) in mixtures due to the masking effect on ARDIs exerted by the presence of conventional aldose reductase inhibitors. The possibility of differential inhibition generated by a different inhibitory model of action of molecules on different substrates undergoing transformation is also discussed.
Rapid characterisation and comparison of saponin profiles in the seeds of Korean Leguminous species using ultra performance liquid chromatography with photodiode array detector and electrospray ionisation/mass spectrometry (UPLC-PDA-ESI/MS) analysis.[Pubmed:24176342]
Food Chem. 2014 Mar 1;146:270-7.
The present work was reported on investigation of saponin profiles in nine different legume seeds, including soybean, adzuki bean, cowpea, common bean, scarlet runner bean, lentil, chick pea, hyacinth bean, and broad bean using ultra performance liquid chromatography with photodiode array detector and electrospray ionisation/mass spectrometry (UPLC-PDA-ESI/MS) technique. A total of twenty saponins were characterised under rapid and simple conditions within 15min by the 80% methanol extracts of all species. Their chemical structures were elucidated as soyasaponin Ab (1), soyasaponin Ba (2), soyasaponin Bb (3), soyasaponin Bc (4), Soyasaponin Bd (5), soyasaponin alphag (6), soyasaponin betag (7), soyasaponin betaa (8), soyasaponin gammag (9), soyasaponin gammaa (10), azukisaponin VI (11), azukisaponin IV (12), azukisaponin II (13), AzII (14), AzIV (15), lablaboside E (16), lablaboside F (17), lablaboside D (18), chikusetusaponin IVa (19), and lablab saponin I (20). The individual and total saponin compositions exhibited remarkable differences in all legume seeds. In particular, soyasaponin betaa (8) was detected the predominant composition in soybean, cowpea, and lentil with various concentrations. Interestingly, soybean, adzuki bean, common bean, and scarlet runner bean had high saponin contents, while chick pea and broad bean showed low contents.
Metabolomics revealed novel isoflavones and optimal cultivation time of Cordyceps militaris fermentation.[Pubmed:20225861]
J Agric Food Chem. 2010 Apr 14;58(7):4258-67.
Germinated soybean (GS) cultivated with Cordyceps militaris (GSC) might be a promising efficacious source of novel bioactive compounds. In this study, the metabolome changes between GS and GSC were investigated by liquid chromatography-mass spectrometry (LC-MS) and gas chromatography-mass spectrometry (GC-MS) analysis coupled with a multivariate data set. Principal component analysis (PCA) and orthogonal projection to latent structures discriminate analysis (OPLS-DA) of GSC clearly showed higher levels of Soyasaponin Bd, soyasaponin Bc(II), daidzein, genistein, four isoflavones (compounds 1-4), glycerol, proline, glutamine, pentitol, fructose, inositol, octadecanoic acid, and sucrose together with lower levels of pyroglutamic acid, citric acid, histidine, and palmitic acid in GSC than in GS. The structures of compounds 1-4 were analyzed by mass and NMR spectroscopy and were determined to be novel isoflavone methyl-glycosides (daidzein 7-O-beta-d-glucoside 4''-O-methylate (1), glycitein 7-O-beta-d-glucoside 4''-O-methylate (2), genistein 7-O-beta-d-glucoside 4''-O-methylate (3), and genistein 4'-O-beta-d-glucoside 4''-O-methylate (4)). Multivariate statistical models showed that metabolic changes of GSC were maximal within 1 week after the C. militaris inoculation, consistent with the strongest antioxidant activity of GSC cultivated for 1 week. This metabolomics study provides valuable information in regard to optimizing the cultivation process for bioactive compound production and describes an efficient way to screen for novel bioactive compounds from GSC.


