CE3F4CAS# 143703-25-7 |
- LDC000067
Catalog No.:BCC5452
CAS No.:1073485-20-7
- CDK9 inhibitor 2
Catalog No.:BCC1466
CAS No.:1263369-28-3
- LY2857785
Catalog No.:BCC8050
CAS No.:1619903-54-6
- PHA-793887
Catalog No.:BCC2521
CAS No.:718630-59-2
- Dinaciclib (SCH727965)
Catalog No.:BCC3765
CAS No.:779353-01-4
- P276-00
Catalog No.:BCC4415
CAS No.:920113-03-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 143703-25-7 | SDF | Download SDF |
| PubChem ID | 21781066 | Appearance | Powder |
| Formula | C11H10Br2FNO | M.Wt | 351.01 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
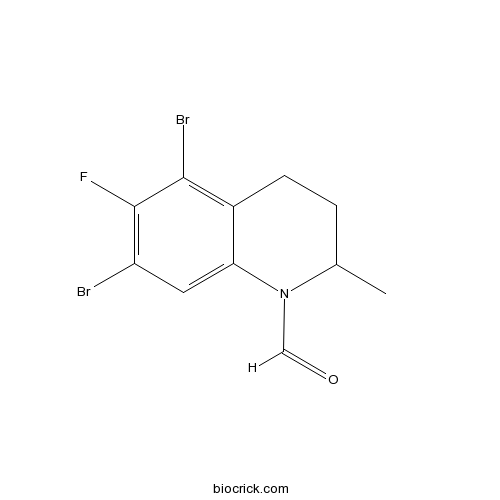
| Chemical Name | 5,7-dibromo-6-fluoro-2-methyl-3,4-dihydro-2H-quinoline-1-carbaldehyde | ||
| SMILES | CC1CCC2=C(C(=C(C=C2N1C=O)Br)F)Br | ||
| Standard InChIKey | ZZLQPWXVZCPUGC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H10Br2FNO/c1-6-2-3-7-9(15(6)5-16)4-8(12)11(14)10(7)13/h4-6H,2-3H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Noncompetitive Epac1 inhibitor. Blocks Epac-induced Rap activation and prevents isoprenaline-induced autophagy flux in cardiomyocytes. Has no effect on PKA activity in the presence of cAMP. |

CE3F4 Dilution Calculator

CE3F4 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8489 mL | 14.2446 mL | 28.4892 mL | 56.9784 mL | 71.223 mL |
| 5 mM | 0.5698 mL | 2.8489 mL | 5.6978 mL | 11.3957 mL | 14.2446 mL |
| 10 mM | 0.2849 mL | 1.4245 mL | 2.8489 mL | 5.6978 mL | 7.1223 mL |
| 50 mM | 0.057 mL | 0.2849 mL | 0.5698 mL | 1.1396 mL | 1.4245 mL |
| 100 mM | 0.0285 mL | 0.1424 mL | 0.2849 mL | 0.5698 mL | 0.7122 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- GYKI 53655 hydrochloride
Catalog No.:BCC7407
CAS No.:143692-48-2
- Fmoc-D-Ile-OPfp
Catalog No.:BCC3507
CAS No.:143688-83-9
- G-Protein antagonist peptide
Catalog No.:BCC7206
CAS No.:143675-79-0
- Elacridar
Catalog No.:BCC1546
CAS No.:143664-11-3
- Diacetoxy-6-gingerdiol
Catalog No.:BCN3339
CAS No.:143615-75-2
- (2R,3S)-3-Phenylisoserine ethyl ester
Catalog No.:BCC8388
CAS No.:143615-00-3
- 3-(4-Chlorobutyl)indole-5-carbonitrile
Catalog No.:BCC8589
CAS No.:143612-79-7
- Curculigoside B
Catalog No.:BCN7939
CAS No.:143601-09-6
- 5-Hydroxy-2-(4-methoxyphenyl)-8,8-dimethyl-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxypyrano[2,3-h]chromen-4-one
Catalog No.:BCC8807
CAS No.:143601-07-4
- Cyclo(Leu-Leu)
Catalog No.:BCN2433
CAS No.:1436-27-7
- A 419259 trihydrochloride
Catalog No.:BCC4308
CAS No.:1435934-25-0
- H-DL-Asp(OMe)-OMe.HCl
Catalog No.:BCC2901
CAS No.:14358-33-9
- Soyasaponin Bd
Catalog No.:BCN2465
CAS No.:135272-91-2
- Kaempferol 3,4,7-triacetate
Catalog No.:BCN6242
CAS No.:143724-69-0
- Pyrazine-2-carbaldehyde
Catalog No.:BCN2565
CAS No.:5780-66-5
- PACAP 6-38
Catalog No.:BCC7611
CAS No.:143748-18-9
- (RS)-Abscisic acid
Catalog No.:BCN8353
CAS No.:14375-45-2
- 3-O-Coumaroylasiatic acid
Catalog No.:BCN7132
CAS No.:143773-52-8
- SB 200646 hydrochloride
Catalog No.:BCC5751
CAS No.:143797-62-0
- 13-Epimanool
Catalog No.:BCN4862
CAS No.:1438-62-6
- 22-Dehydroclerosterol glucoside
Catalog No.:BCN6243
CAS No.:143815-99-0
- Fmoc-Trp(Boc)-OH
Catalog No.:BCC3558
CAS No.:143824-78-6
- 2,24-Dihydroxyursolic acid
Catalog No.:BCN6244
CAS No.:143839-02-5
- CC-401 hydrochloride
Catalog No.:BCC1458
CAS No.:1438391-30-0
The (R)-enantiomer of CE3F4 is a preferential inhibitor of human exchange protein directly activated by cyclic AMP isoform 1 (Epac1).[Pubmed:24099776]
Biochem Biophys Res Commun. 2013 Oct 25;440(3):443-8.
Isoform 1 and isoform 2 of exchange protein directly activated by cAMP (Epac1 and Epac2) contribute to cAMP signaling in numerous cellular processes. Their guanine-nucleotide exchange factor (GEF) activity toward the small GTP-binding protein Rap1 is stimulated by the agonist cAMP. CE3F4, a tetrahydroquinoline analog, prevents Epac1 activation in vitro and in living cultured cells by inhibiting the GEF activity of Epac1. However, the activity of the (R)- and (S)-enantiomers of CE3F4, as well as the ability of CE3F4 and its analogs to inhibit Epac2 GEF activity, have not yet been investigated. In this study, we report that (R)-CE3F4 is a more potent cAMP antagonist than racemic CE3F4 and (S)-CE3F4, inhibiting the GEF activity of Epac1 with 10-times more efficiency than (S)-CE3F4. Epac2, in contrast to Epac1, is activated more efficiently by cAMP than by 8-(4-chlorophenylthio)-2'-O-methyladenosine-3',5'-cyclic monophosphate (007), an Epac-selective cAMP analog. (R)-CE3F4 displays Epac isoform preference, with 10-fold selectivity for Epac1 over Epac2. Deletion of the N-terminal cyclic nucleotide-binding domain of Epac2 does not affect the characteristics of activation of Epac2 by cAMP and by 007, nor its inhibition by CE3F4. Finally, the evaluation of a series of CE3F4 structural analogs as GEF inhibitors allowed identifying structural features that are important for high Epac1 inhibitory activity of CE3F4. We conclude that the (R)-enantiomer of CE3F4 is a preferential inhibitor of Epac1 with high potency in the low micromolar range, and we suggest that this compound may be a useful pharmacological tool for investigating the functional role of Epac1 in cAMP signaling.


