ErythristemineCAS# 28619-41-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 28619-41-2 | SDF | Download SDF |
| PubChem ID | 12143915 | Appearance | Powder |
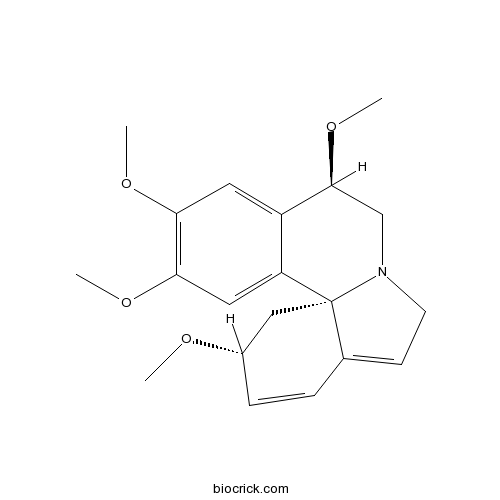
| Formula | C20H25NO4 | M.Wt | 343.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R,9R,13bS)-2,9,11,12-tetramethoxy-2,6,8,9-tetrahydro-1H-indolo[7a,1-a]isoquinoline | ||
| SMILES | COC1CC23C(=CCN2CC(C4=CC(=C(C=C34)OC)OC)OC)C=C1 | ||
| Standard InChIKey | IUMRZRWBQPPMSS-GKCIPKSASA-N | ||
| Standard InChI | InChI=1S/C20H25NO4/c1-22-14-6-5-13-7-8-21-12-19(25-4)15-9-17(23-2)18(24-3)10-16(15)20(13,21)11-14/h5-7,9-10,14,19H,8,11-12H2,1-4H3/t14-,19-,20-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. (+)-Erythristemine shows moderate toxicity to brine shrimp (LC50 23 ppm) and moderate (IC50=86microg/ml) radical scavenging properties against stable 2,2-diphenyl-1-picrylhy--drazyl (DPPH) radical. |

Erythristemine Dilution Calculator

Erythristemine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9121 mL | 14.5603 mL | 29.1206 mL | 58.2411 mL | 72.8014 mL |
| 5 mM | 0.5824 mL | 2.9121 mL | 5.8241 mL | 11.6482 mL | 14.5603 mL |
| 10 mM | 0.2912 mL | 1.456 mL | 2.9121 mL | 5.8241 mL | 7.2801 mL |
| 50 mM | 0.0582 mL | 0.2912 mL | 0.5824 mL | 1.1648 mL | 1.456 mL |
| 100 mM | 0.0291 mL | 0.1456 mL | 0.2912 mL | 0.5824 mL | 0.728 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 8-Prenylkaempferol
Catalog No.:BCN3311
CAS No.:28610-31-3
- Isoanhydroicaritin
Catalog No.:BCN3879
CAS No.:28610-30-2
- Orientin
Catalog No.:BCN4984
CAS No.:28608-75-5
- CCG-1423
Catalog No.:BCC5581
CAS No.:285986-88-1
- BIRB 796 (Doramapimod)
Catalog No.:BCC2535
CAS No.:285983-48-4
- 22-Dehydroclerosteryl acetate
Catalog No.:BCN5183
CAS No.:28594-00-5
- Docosyl caffeate
Catalog No.:BCN5182
CAS No.:28593-92-2
- Eicosanyl caffeate
Catalog No.:BCN7209
CAS No.:28593-90-0
- Persicogenin
Catalog No.:BCN7744
CAS No.:28590-40-1
- 7-Geranyloxy-6-methoxycoumarin
Catalog No.:BCN5181
CAS No.:28587-43-1
- 4-Benzoyloxy-2-azetidinone
Catalog No.:BCC8696
CAS No.:28562-58-5
- Theaflavin-3'-gallate
Catalog No.:BCN5421
CAS No.:28543-07-9
- S 14506 hydrochloride
Catalog No.:BCC7174
CAS No.:286369-38-8
- KRN 633
Catalog No.:BCC2544
CAS No.:286370-15-8
- Nigericin sodium salt
Catalog No.:BCC7915
CAS No.:28643-80-3
- Meloscandonine
Catalog No.:BCN5186
CAS No.:28645-27-4
- L-838,417
Catalog No.:BCC7617
CAS No.:286456-42-6
- Multicaulisin
Catalog No.:BCN7840
CAS No.:286461-76-5
- Euphorbiasteroid
Catalog No.:BCN2781
CAS No.:28649-59-4
- Epoxylathyrol
Catalog No.:BCN5382
CAS No.:28649-60-7
- 6'-Amino-3',4'-(methylenedioxy)acetophenone
Catalog No.:BCC8760
CAS No.:28657-75-2
- Fesoterodine Fumarate
Catalog No.:BCC4584
CAS No.:286930-03-8
- Ezatiostat hydrochloride
Catalog No.:BCC4259
CAS No.:286942-97-0
- NCX 4040
Catalog No.:BCC7944
CAS No.:287118-97-2
Erythrinaline alkaloids from the flowers and pods of Erythrina lysistemon and their DPPH radical scavenging properties.[Pubmed:15231413]
Phytochemistry. 2004 May;65(10):1397-404.
Fourteen different erythrinaline alkaloids have been isolated from the flowers and pods of Erythrina lysistemon with four being reported for the first time in nature and five for the first time in this species and the rest having been re-isolated. The new compounds are (+)-11beta-hydroxyerysotramidine (1), (+)-11beta-methoxyerysotramidine (2), (+)-11beta-hydroxyerysotrine N-oxide (4) and (+)-11beta-hydroxyerysotrine (8). (+)-11alpha-Hydroxyerysotrine N-oxide (3), earlier misidentified as erythrartine N-oxide (beta-hydroxyerysotrine N-oxide 4), was also re-isolated along with four other alkaloids. Correct identification of compounds 4 and 8 was aided by the fact that the two sets of C-11 epimers 3, 4 and 8, 9 were both isolated in this study thus making it easier to identify and assign the individual epimers. (+)-Erythristemine (14) was found distributed in most of the plant parts investigated. Preliminary work on the crude chloroform/methanol (1:1) showed moderate toxicity to brine shrimp (LC50 23 ppm) and moderate (IC50 86 microg/ml) radical scavenging properties against stable 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical. The DPPH radical scavenging properties of the isolated compounds were assessed using TLC autographic and spectrophotometric assays whereupon only compounds 11 (1 microg; 90 microg/ml) and 12 (0.1 microg; 160 microg/ml) showed any notable activity. It appears the two compounds are slow reacting and do not reach steady state conditions within the standard half an hour time frame but only seemed to have reached steady state conditions after 4 h.


