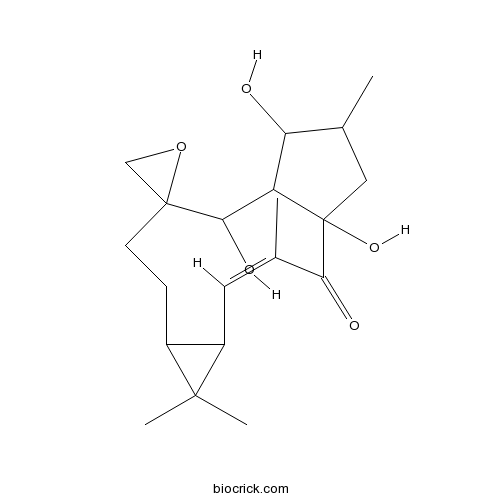
EpoxylathyrolCAS# 28649-60-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 28649-60-7 | SDF | Download SDF |
| PubChem ID | 6443462 | Appearance | Powder |
| Formula | C20H30O5 | M.Wt | 350.45 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CC1CC2(C(C1O)C(C3(CCC4C(C4(C)C)C=C(C2=O)C)CO3)O)O | ||
| Standard InChIKey | VEFQDSXSELSHMX-YFHOEESVSA-N | ||
| Standard InChI | InChI=1S/C20H30O5/c1-10-7-13-12(18(13,3)4)5-6-19(9-25-19)17(23)14-15(21)11(2)8-20(14,24)16(10)22/h7,11-15,17,21,23-24H,5-6,8-9H2,1-4H3/b10-7- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Epoxylathyrol derivatives have modulation of ABCB1-mediated multidrug resistance in human colon adenocarcinoma and mouse T-Lymphoma cells. |

Epoxylathyrol Dilution Calculator

Epoxylathyrol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8535 mL | 14.2674 mL | 28.5347 mL | 57.0695 mL | 71.3369 mL |
| 5 mM | 0.5707 mL | 2.8535 mL | 5.7069 mL | 11.4139 mL | 14.2674 mL |
| 10 mM | 0.2853 mL | 1.4267 mL | 2.8535 mL | 5.7069 mL | 7.1337 mL |
| 50 mM | 0.0571 mL | 0.2853 mL | 0.5707 mL | 1.1414 mL | 1.4267 mL |
| 100 mM | 0.0285 mL | 0.1427 mL | 0.2853 mL | 0.5707 mL | 0.7134 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Euphorbiasteroid
Catalog No.:BCN2781
CAS No.:28649-59-4
- Multicaulisin
Catalog No.:BCN7840
CAS No.:286461-76-5
- L-838,417
Catalog No.:BCC7617
CAS No.:286456-42-6
- Meloscandonine
Catalog No.:BCN5186
CAS No.:28645-27-4
- Nigericin sodium salt
Catalog No.:BCC7915
CAS No.:28643-80-3
- KRN 633
Catalog No.:BCC2544
CAS No.:286370-15-8
- S 14506 hydrochloride
Catalog No.:BCC7174
CAS No.:286369-38-8
- Erythristemine
Catalog No.:BCN5184
CAS No.:28619-41-2
- 8-Prenylkaempferol
Catalog No.:BCN3311
CAS No.:28610-31-3
- Isoanhydroicaritin
Catalog No.:BCN3879
CAS No.:28610-30-2
- Orientin
Catalog No.:BCN4984
CAS No.:28608-75-5
- CCG-1423
Catalog No.:BCC5581
CAS No.:285986-88-1
- 6'-Amino-3',4'-(methylenedioxy)acetophenone
Catalog No.:BCC8760
CAS No.:28657-75-2
- Fesoterodine Fumarate
Catalog No.:BCC4584
CAS No.:286930-03-8
- Ezatiostat hydrochloride
Catalog No.:BCC4259
CAS No.:286942-97-0
- NCX 4040
Catalog No.:BCC7944
CAS No.:287118-97-2
- PD 180970
Catalog No.:BCC3894
CAS No.:287204-45-9
- MA 2029
Catalog No.:BCC7983
CAS No.:287206-61-5
- Oxcarbazepine
Catalog No.:BCC5077
CAS No.:28721-07-5
- Scriptaid
Catalog No.:BCC2163
CAS No.:287383-59-9
- 3CAI
Catalog No.:BCC5402
CAS No.:28755-03-5
- Apigenin 5-O-beta-D-glucopyranoside
Catalog No.:BCN5185
CAS No.:28757-27-9
- Rosuvastatin
Catalog No.:BCC4139
CAS No.:287714-41-4
- 4,5,6,7-Tetrahydrothieno [3,2,c]pyridine hydrochloride
Catalog No.:BCC8664
CAS No.:28783-41-7
Epoxylathyrol Derivatives: Modulation of ABCB1-Mediated Multidrug Resistance in Human Colon Adenocarcinoma and Mouse T-Lymphoma Cells.[Pubmed:26331763]
J Nat Prod. 2015 Sep 25;78(9):2215-28.
Epoxyboetirane A (1), a macrocyclic diterpene that was found to be inactive as an ABCB1 modulator, was submitted to several chemical transformations, aimed at generating a series of compounds with improved multidrug resistance (MDR)-modifying activity. Overall, 23 new derivatives were prepared, in addition to the already reported Epoxylathyrol (2) and methoxyboetirol (3). Their anti-MDR potential was assessed through both functional and chemosensitivity assays on resistant human colon adenocarcinoma and human ABCB1-gene transfected L5178Y mouse lymphoma cells. Structure-activity relationship analysis showed that different substitution patterns led to distinct ABCB1 inhibitory activities, although intrinsic cellular characteristics seemed to influence the modulatory behavior. A considerable enhancement in MDR-modifying activity was observed for aromatic compounds in both cell lines, particularly in 3,17-disubstituted esters derived from 3, a Payne-rearranged Michael adduct of 2. All compounds tested were revealed to interact synergistically with doxorubicin, and ATPase inhibition by three representative MDR-modifying compounds was also investigated. On account of its outstanding ABCB1 inhibitory activity at 0.2 muM and overall remarkable bioactive profile, methoxyboetirane B (22) was found to be a new promising lead for MDR-reversing anticancer drug development.


