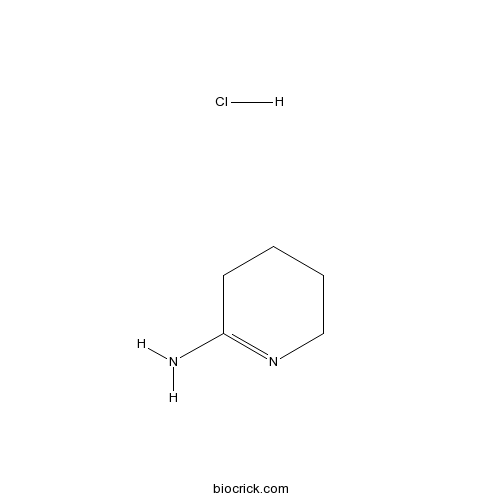
2-Iminopiperidine hydrochlorideSelective iNOS inhibitor CAS# 16011-96-4 |
- Thrombin Receptor Agonist Peptide
Catalog No.:BCC3950
CAS No.:137339-65-2
- SLIGRL-NH2
Catalog No.:BCC3947
CAS No.:171436-38-7
- TFLLR-NH2
Catalog No.:BCC3948
CAS No.:197794-83-5
- AY-NH2
Catalog No.:BCC3949
CAS No.:352017-71-1
- ML161
Catalog No.:BCC3642
CAS No.:423735-93-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 16011-96-4 | SDF | Download SDF |
| PubChem ID | 85236 | Appearance | Powder |
| Formula | C5H11ClN2 | M.Wt | 134.61 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
| Chemical Name | 2,3,4,5-tetrahydropyridin-6-amine;hydrochloride | ||
| SMILES | C1CCN=C(C1)N.Cl | ||
| Standard InChIKey | ZHDTXTDHBRADLM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C5H10N2.ClH/c6-5-3-1-2-4-7-5;/h1-4H2,(H2,6,7);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | An inhibitor of NO synthases, more potent than NG-methyl-L-arginine and selective for iNOS. |

2-Iminopiperidine hydrochloride Dilution Calculator

2-Iminopiperidine hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.4289 mL | 37.1443 mL | 74.2887 mL | 148.5774 mL | 185.7217 mL |
| 5 mM | 1.4858 mL | 7.4289 mL | 14.8577 mL | 29.7155 mL | 37.1443 mL |
| 10 mM | 0.7429 mL | 3.7144 mL | 7.4289 mL | 14.8577 mL | 18.5722 mL |
| 50 mM | 0.1486 mL | 0.7429 mL | 1.4858 mL | 2.9715 mL | 3.7144 mL |
| 100 mM | 0.0743 mL | 0.3714 mL | 0.7429 mL | 1.4858 mL | 1.8572 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- SCH 58261
Catalog No.:BCC7306
CAS No.:160098-96-4
- Cryptofolione
Catalog No.:BCN7197
CAS No.:160098-78-2
- Huwentoxin XVI
Catalog No.:BCC8041
CAS No.:1600543-88-1
- Sambutoxin
Catalog No.:BCN1709
CAS No.:160047-56-3
- Antibiotic AB 4063B
Catalog No.:BCN1827
CAS No.:160041-33-8
- Iniparib (BSI-201)
Catalog No.:BCC2208
CAS No.:160003-66-7
- Nelfinavir Mesylate
Catalog No.:BCC1794
CAS No.:159989-65-8
- Nelfinavir
Catalog No.:BCC4138
CAS No.:159989-64-7
- NIBR189
Catalog No.:BCC8056
CAS No.:1599432-08-2
- 3,5-Di-O-caffeoylquinic acid methyl ester
Catalog No.:BCN6491
CAS No.:159934-13-1
- Mc-Val-Cit-PABC-PNP
Catalog No.:BCC4028
CAS No.:159857-81-5
- 4'-O-Methylirenolone
Catalog No.:BCN7174
CAS No.:159853-36-8
- L-BMAA hydrochloride
Catalog No.:BCC7400
CAS No.:16012-55-8
- 7-Chloro-1,2,3,4-tetrahydrobenzo[b]azepin-5-one
Catalog No.:BCC8779
CAS No.:160129-45-3
- BIM 23127
Catalog No.:BCC5822
CAS No.:160161-61-5
- SR 11302
Catalog No.:BCC3607
CAS No.:160162-42-5
- 14-Deoxy-11-hydroxyandrographolide
Catalog No.:BCN4702
CAS No.:160242-09-1
- SB 205384
Catalog No.:BCC7095
CAS No.:160296-13-9
- 8-Hydroxybergapten
Catalog No.:BCN2732
CAS No.:1603-47-0
- L-368,899 hydrochloride
Catalog No.:BCC7438
CAS No.:160312-62-9
- Bisdehydroneotuberostemonine
Catalog No.:BCN7072
CAS No.:160333-27-7
- X-NeuNAc
Catalog No.:BCC2063
CAS No.:160369-85-7
- 3',5,5',7-Tetrahydroxyflavanone
Catalog No.:BCN1710
CAS No.:160436-10-2
- 30-Oxopseudotaraxasterol
Catalog No.:BCN7135
CAS No.:160481-71-0
Selective inhibitors of neuronal nitric oxide synthase--is no NOS really good NOS for the nervous system?[Pubmed:9226999]
Trends Pharmacol Sci. 1997 Jun;18(6):204-11.
It is now ten years since NO was shown to account for the biological activity of endothelium-derived relaxing factor (EDRF). It is also the tenth anniversary of the identification of L-NG monomethyl arginine (L-NMMA) as the very first inhibitor of NO biosynthesis. That EDRF and NO were one and the same sparked an explosion of interest in the biochemistry and pharmacology of NO which has yet to subside. In contrast, the first ever nitric oxide synthase (NOS) inhibitor slipped seamlessly into the literature virtually without comment at the time. Over the following decade, L-NMMA (and like NOS inhibitors) have proved invaluable as tools for probing the biological roles of NO in health and disease and, in particular, have increased our understanding of the function of NO in the nervous system. Further advances in this important area now require the development of inhibitors selective for the neuronal isoform of NOS (nNOS). Here, Philip Moore and Rachel Handy provide an up-to-date account of the literature regarding the biochemical and pharmacological characterization of NOS inhibitors with particular reference to compounds with greater selectivity for the nNOS isoform.
2-Iminopiperidine and other 2-iminoazaheterocycles as potent inhibitors of human nitric oxide synthase isoforms.[Pubmed:8576908]
J Med Chem. 1996 Feb 2;39(3):669-72.
A series of 2-iminoazaheterocycles have been prepared and shown to be potent inhibitors of human nitric oxide synthase (NOS) isoforms. This series includes cyclic amidines ranging from five- to nine-membered rings, of which 2-iminopiperidine and 2-iminohomopiperidine were the most potent inhibitors, with IC50 values of 1.0 and 2.0 microM, respectively, for human inducible nitric oxide synthase. This series of cyclic inhibitors was further expanded to include analogs with heteroatoms in the 3-position of the six-membered ring. This modification was tolerated for sulfur and oxygen, but nitrogen reduced the inhibitory potency. The oral administration of 2-iminopiperidine in lipopolysaccharide (LPS)-treated rats inhibited the LPS-induced increase in plasma nitrite/nitrate levels in a dose-dependent manner, demonstrating its ability to inhibit inducible NOS activity in vivo. These cyclic amidines represent a new class of potent NOS inhibitors and the foundation for potential therapeutic agents.
Amidines are potent inhibitors of nitric oxide synthases: preferential inhibition of the inducible isoform.[Pubmed:8719415]
Eur J Pharmacol. 1995 Nov 30;291(3):311-8.
We evaluated the ability of simple alkyl amidines to inhibit the activity of the inducible isoform of nitric oxide (NO) synthase in vitro. In immunostimulated J774 macrophages, 2-iminopiperidine (EC50 = 10 microM) and butyramidine (EC50 = 60 microM) were more potent than NG-methyl-L-arginine (EC50 = 70 microM) in inhibiting nitrite formation. The five amidines tested for their ability to inhibit the conversion of L-arginine to L-citrulline by bovine endothelial cell homogenates (a source of the constitutive, endothelial NO synthase isoform) were less effective than NG-nitro-L-arginine or NG-methyl-L-arginine. The rank-order of the potencies of the amidines against the endothelial NO synthase was, in general, similar to the rank-order of the pressor effects of these agents in anesthetized rats. Thus, certain amidines are potent inhibitors of NO synthase, and are more selective towards the inducible NO synthase than the commonly used L-arginine based NO synthase inhibitors.


