L-368,899 hydrochloridePotent, non-peptide oxytocin receptor antagonist CAS# 160312-62-9 |
- GW1929
Catalog No.:BCC1611
CAS No.:196808-24-9
- Balaglitazone
Catalog No.:BCC1395
CAS No.:199113-98-9
- Inolitazone
Catalog No.:BCC1652
CAS No.:223132-37-4
- Inolitazone dihydrochloride
Catalog No.:BCC1653
CAS No.:223132-38-5
- Aleglitazar
Catalog No.:BCC1337
CAS No.:475479-34-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 160312-62-9 | SDF | Download SDF |
| PubChem ID | 90488775 | Appearance | Powder |
| Formula | C26H43ClN4O5S2 | M.Wt | 591.23 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 130 mg/mL (219.88 mM; Need ultrasonic) | ||
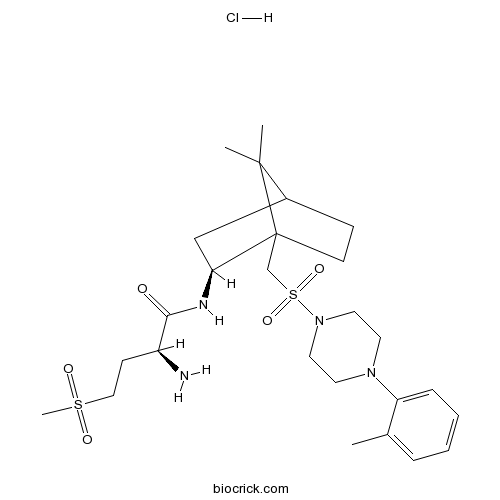
| Chemical Name | (2S)-2-amino-N-[(2S)-7,7-dimethyl-1-[[4-(2-methylphenyl)piperazin-1-yl]sulfonylmethyl]-2-bicyclo[2.2.1]heptanyl]-4-methylsulfonylbutanamide;hydrochloride | ||
| SMILES | CC1=CC=CC=C1N2CCN(CC2)S(=O)(=O)CC34CCC(C3(C)C)CC4NC(=O)C(CCS(=O)(=O)C)N.Cl | ||
| Standard InChIKey | GIUFQWFJHXXXEQ-PHSYAEQHSA-N | ||
| Standard InChI | InChI=1S/C26H42N4O5S2.ClH/c1-19-7-5-6-8-22(19)29-12-14-30(15-13-29)37(34,35)18-26-11-9-20(25(26,2)3)17-23(26)28-24(31)21(27)10-16-36(4,32)33;/h5-8,20-21,23H,9-18,27H2,1-4H3,(H,28,31);1H/t20?,21-,23-,26?;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, non-peptide and orally active oxytocin receptor antagonist (IC50 = 8.9 nM) that displays > 40-fold selectivity over vasopressin V1a and V2 receptors (IC50 values are 370 and 570 nM respectively). Antagonizes oxytocin-induced uterine contractions in vitro and in vivo. |

L-368,899 hydrochloride Dilution Calculator

L-368,899 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6914 mL | 8.4569 mL | 16.9139 mL | 33.8278 mL | 42.2847 mL |
| 5 mM | 0.3383 mL | 1.6914 mL | 3.3828 mL | 6.7656 mL | 8.4569 mL |
| 10 mM | 0.1691 mL | 0.8457 mL | 1.6914 mL | 3.3828 mL | 4.2285 mL |
| 50 mM | 0.0338 mL | 0.1691 mL | 0.3383 mL | 0.6766 mL | 0.8457 mL |
| 100 mM | 0.0169 mL | 0.0846 mL | 0.1691 mL | 0.3383 mL | 0.4228 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 8-Hydroxybergapten
Catalog No.:BCN2732
CAS No.:1603-47-0
- SB 205384
Catalog No.:BCC7095
CAS No.:160296-13-9
- 14-Deoxy-11-hydroxyandrographolide
Catalog No.:BCN4702
CAS No.:160242-09-1
- SR 11302
Catalog No.:BCC3607
CAS No.:160162-42-5
- BIM 23127
Catalog No.:BCC5822
CAS No.:160161-61-5
- 7-Chloro-1,2,3,4-tetrahydrobenzo[b]azepin-5-one
Catalog No.:BCC8779
CAS No.:160129-45-3
- L-BMAA hydrochloride
Catalog No.:BCC7400
CAS No.:16012-55-8
- 2-Iminopiperidine hydrochloride
Catalog No.:BCC6862
CAS No.:16011-96-4
- SCH 58261
Catalog No.:BCC7306
CAS No.:160098-96-4
- Cryptofolione
Catalog No.:BCN7197
CAS No.:160098-78-2
- Huwentoxin XVI
Catalog No.:BCC8041
CAS No.:1600543-88-1
- Sambutoxin
Catalog No.:BCN1709
CAS No.:160047-56-3
- Bisdehydroneotuberostemonine
Catalog No.:BCN7072
CAS No.:160333-27-7
- X-NeuNAc
Catalog No.:BCC2063
CAS No.:160369-85-7
- 3',5,5',7-Tetrahydroxyflavanone
Catalog No.:BCN1710
CAS No.:160436-10-2
- 30-Oxopseudotaraxasterol
Catalog No.:BCN7135
CAS No.:160481-71-0
- THZ1
Catalog No.:BCC4005
CAS No.:1604810-83-4
- THZ2
Catalog No.:BCC3986
CAS No.:1604810-84-5
- Antirhine
Catalog No.:BCN4003
CAS No.:16049-28-8
- Bisandrographolide A
Catalog No.:BCN4701
CAS No.:160498-00-0
- 12S-hydroxyandrographolide
Catalog No.:BCN4700
CAS No.:869593-50-0
- BW 723C86 hydrochloride
Catalog No.:BCC6915
CAS No.:160521-72-2
- 5,7,3',4'-Tetrahydroxy-3-methoxy-8-geranylflavone
Catalog No.:BCN6847
CAS No.:1605304-56-0
- Zarzissine
Catalog No.:BCN6456
CAS No.:160568-14-9
Discovery and development of a new class of potent, selective, orally active oxytocin receptor antagonists.[Pubmed:16302826]
J Med Chem. 2005 Dec 1;48(24):7882-905.
We report a novel chemical class of potent oxytocin receptor antagonists showing a high degree of selectivity against the closely related vasopressin receptors (V1a, V1b, V2). An initial compound, 7, was shown to be active in an animal model of preterm labor when administered by the intravenous but not by the oral route. Stepwise SAR investigations around the different structural elements revealed one position, the arenesulfonyl moiety, to be amenable to structural changes. Consequently, this position was used to introduce a variety of substituents to improve the physicochemical properties. Some of the resulting analogues were found to be superior to 7 both in terms of potency in vitro and aqueous solubility, which translated into significantly improved efficacy in the animal model after intravenous and oral administration. The best compound, 73, potently inhibited oxytocin-induced uterine contractions in nonpregnant rats and reduced spontaneous uterine contractions in late-term pregnant rats.
Attenuation of PGF2alpha release in ewes infused with the oxytocin antagonist L-368,899.[Pubmed:14550509]
Domest Anim Endocrinol. 2003 Oct;25(3):255-62.
We have investigated the effects of systemic administration of the oxytocin antagonist (OTA) L-368,899 on luteolytic PGF(2alpha) release in ewes. In the first study, carried out in four ovariectomized ewes primed with progesterone to induce responsiveness to oxytocin, 3-h i.v. infusions of 3, 10 and 30 microg/kg/min OTA, carried out on days 12, 14, 16 and 18 in a Latin Square design, resulted in a significant attenuation of the oxytocin induced increase in PGFM concentration at all doses (OTA 139+/-8.3% of pre-oxytocin baseline; control 206.8+/-18.7%; P<0.005). In a further study, continuous infusion of cyclic ewes (n=6) with 10 microg/kg/min OTA from day 13 to day 17 of the cycle resulted in a reduction in both the frequency (OTA 1.0+/-0.4/ewe; control 2.2+/-0.2/ewe; P<0.05) and amplitude (OTA 31.8+/-11.0 pg/ml; control 68.8+/-10.4 pg/ml; P<0.05) of endogenous PGFM episodes compared to control ewes (n=5) measured during daily 8-h sampling windows on days 14-17. This reduction in PGFM concentrations was accompanied by a modest extension in the day of luteolysis (progesterone <0.5 ng/ml) to day 17.5+/-0.4 in the OTA treated group compared with day 16.4+/-0.5 in the control group (P=0.07). The results demonstrate that treatment with OTA caused a significant reduction in episodes of increased PGFM concentration during the period of luteolysis and may provide an approach by which to reduce early pregnancy failure.
1-((7,7-Dimethyl-2(S)-(2(S)-amino-4-(methylsulfonyl)butyramido)bicyclo [2.2.1]-heptan-1(S)-yl)methyl)sulfonyl)-4-(2-methylphenyl)piperaz ine (L-368,899): an orally bioavailable, non-peptide oxytocin antagonist with potential utility for managing preterm labor.[Pubmed:8126695]
J Med Chem. 1994 Mar 4;37(5):565-71.
Modifications to the previously reported spiroindenylpiperidine camphor-sulfonamide oxytocin (OT) antagonist L-366,509 have produced a new series of o-tolylpiperazine (TP) camphor-sulfonamides. A number of analogues in the TP series that incorporate a modified or unmodified L-methionine sulfone amide at the C2 endo position on the camphor ring exhibit high affinity for OT receptors (IC50 = 1.3-15 nM) and good selectivity for binding to OT versus arginine vasopressin V1a and V2 receptors. Several of these analogues were additionally characterized as potent antagonists of OT-stimulated contractions of the isolated and/or in situ rat uterus. Compound 7 (L-368,899) exhibited the best overall profile of OT receptor affinity (IC50 = 8.9 nM, rat uterus; 26 nM, human uterus), potency for inhibition of OT-stimulated contractions of the isolated rat uterus (pA2 = 8.9) and in situ rat uterus (AD50 = 0.35 mg/kg after intravenous (i.v.) administration and 7.0 mg/kg after intraduodenal administration), aqueous solubility (3.7 mg/mL at pH 5.0), and oral bioavailability in several species (35% (rat), 25% (dog), and 21% (chimpanzee) as estimated from radioreceptor determination of drug levels in plasma after oral and i.v. dosing). On the basis of these favorable properties, 7 has begun clinical testing for use as an oral and i.v. tocolytic agent. Molecular modeling alignment studies have provided support for the hypothesis that the TP camphor-sulfonamide portion of the non-peptide structures may serve as a mimetic of the important D-AA2-Ile3 dipeptide (AA = aromatic amino acid) found in many potent OT antagonists from the cyclic hexapeptide and OT analogue structural classes.


