SR 11302AP-1 transcription factor inhibitor CAS# 160162-42-5 |
- PA-824
Catalog No.:BCC1106
CAS No.:187235-37-6
- Clofazimine
Catalog No.:BCC4651
CAS No.:2030-63-9
- 5-hydroxypyrazine-2-carboxylic acid
Catalog No.:BCC1311
CAS No.:34604-60-9
- Nitazoxanide
Catalog No.:BCC3824
CAS No.:55981-09-4
- Sodium 4-Aminosalicylate
Catalog No.:BCC4609
CAS No.:6018-19-5
- Rifapentine
Catalog No.:BCC4937
CAS No.:61379-65-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 160162-42-5 | SDF | Download SDF |
| PubChem ID | 9976842 | Appearance | Powder |
| Formula | C26H32O2 | M.Wt | 376.54 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 10 mM in ethanol | ||
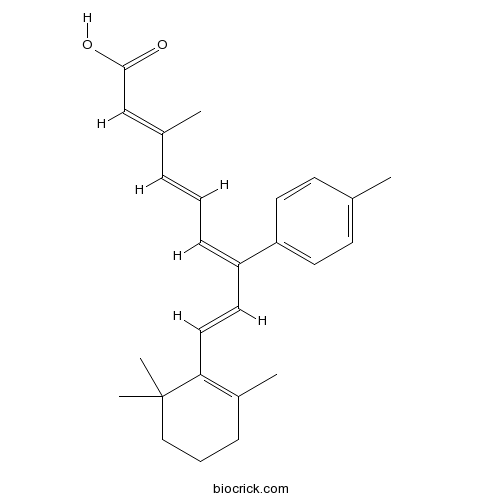
| Chemical Name | (2E,4E,6Z,8E)-3-methyl-7-(4-methylphenyl)-9-(2,6,6-trimethylcyclohexen-1-yl)nona-2,4,6,8-tetraenoic acid | ||
| SMILES | CC1=C(C(CCC1)(C)C)C=CC(=CC=CC(=CC(=O)O)C)C2=CC=C(C=C2)C | ||
| Standard InChIKey | RQANARBNMTXCDM-DKOHIBGUSA-N | ||
| Standard InChI | InChI=1S/C26H32O2/c1-19-11-13-23(14-12-19)22(10-6-8-20(2)18-25(27)28)15-16-24-21(3)9-7-17-26(24,4)5/h6,8,10-16,18H,7,9,17H2,1-5H3,(H,27,28)/b8-6+,16-15+,20-18+,22-10- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of activator protein-1 (AP-1) transcription factor activity that displays antitumor effects in vivo. Does not activate transcription from the retinoic acid response element (RARE) and displays no activity at retinoic acid receptors (EC50 > 1 μM for RARα, RARβ, RARγ and RXRα). |

SR 11302 Dilution Calculator

SR 11302 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6558 mL | 13.2788 mL | 26.5576 mL | 53.1152 mL | 66.394 mL |
| 5 mM | 0.5312 mL | 2.6558 mL | 5.3115 mL | 10.623 mL | 13.2788 mL |
| 10 mM | 0.2656 mL | 1.3279 mL | 2.6558 mL | 5.3115 mL | 6.6394 mL |
| 50 mM | 0.0531 mL | 0.2656 mL | 0.5312 mL | 1.0623 mL | 1.3279 mL |
| 100 mM | 0.0266 mL | 0.1328 mL | 0.2656 mL | 0.5312 mL | 0.6639 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
SR 11302 is an inhibitor of activator protein-1 (AP-1) [1].AP-1 is a transcription factor that displays antitumor effects in vivo.
SR 11302 is an inhibitor of AP-1 but don’t activate RARs and RXR. SR11302 failed to inhibit the proliferation of F9 cells, the embryonal carcinoma cell line. While, it can inhibit the growth of breast cancer cell line T-47D, the lung cancer line Calu-6 and HeLa cells[1]. SR 11302 had very little effect on either the proliferation or the differentiation of HL-60, fresh APL and NB4 cells, which indicate that AP-1 may not be involved in the signaling pathway of proliferation and differentiation of HL-60, fresh APL and NB4 cells [2].
In an AP-1-luciferase transgenic mouse carcinogenesis model, SR11302 significantly inhibit both AP-1 activation in 7,12-dimethyl benz(a)anthracene-initiated mouse skin and 12-O-tetradecanoylphorbol-13-acetate-induced papilloma formation. While, SR11235, a retinoid with RARE transactivating activity, but lack of AP-1 inhibiting effect, didn’t inhibit papilloma formation and AP-1 activation. These results show that the antitumor effect of retinoids is mediated by blocking AP-1 activity in vivo [3].
References:
[1]. Fanjul A, Dawson MI, Hobbs PD, et al. A new class of retinoids with selective inhibition of AP-1 inhibits proliferation. Nature, 1994, 372(6501): 107-111.
[2]. Shiohara M, Dawson MI, Hobbs PD, et al. Effects of novel RAR- and RXR-selective retinoids on myeloid leukemic proliferation and differentiation in vitro. Blood, 1999, 93(6): 2057-2066.
[3]. Huang C, Ma WY, Dawson MI, et al. Blocking activator protein-1 activity, but not activating retinoic acid response element, is required for the antitumor promotion effect of retinoic acid. Proc Natl Acad Sci U S A, 1997, 94(11): 5826-5830.
- BIM 23127
Catalog No.:BCC5822
CAS No.:160161-61-5
- 7-Chloro-1,2,3,4-tetrahydrobenzo[b]azepin-5-one
Catalog No.:BCC8779
CAS No.:160129-45-3
- L-BMAA hydrochloride
Catalog No.:BCC7400
CAS No.:16012-55-8
- 2-Iminopiperidine hydrochloride
Catalog No.:BCC6862
CAS No.:16011-96-4
- SCH 58261
Catalog No.:BCC7306
CAS No.:160098-96-4
- Cryptofolione
Catalog No.:BCN7197
CAS No.:160098-78-2
- Huwentoxin XVI
Catalog No.:BCC8041
CAS No.:1600543-88-1
- Sambutoxin
Catalog No.:BCN1709
CAS No.:160047-56-3
- Antibiotic AB 4063B
Catalog No.:BCN1827
CAS No.:160041-33-8
- Iniparib (BSI-201)
Catalog No.:BCC2208
CAS No.:160003-66-7
- Nelfinavir Mesylate
Catalog No.:BCC1794
CAS No.:159989-65-8
- Nelfinavir
Catalog No.:BCC4138
CAS No.:159989-64-7
- 14-Deoxy-11-hydroxyandrographolide
Catalog No.:BCN4702
CAS No.:160242-09-1
- SB 205384
Catalog No.:BCC7095
CAS No.:160296-13-9
- 8-Hydroxybergapten
Catalog No.:BCN2732
CAS No.:1603-47-0
- L-368,899 hydrochloride
Catalog No.:BCC7438
CAS No.:160312-62-9
- Bisdehydroneotuberostemonine
Catalog No.:BCN7072
CAS No.:160333-27-7
- X-NeuNAc
Catalog No.:BCC2063
CAS No.:160369-85-7
- 3',5,5',7-Tetrahydroxyflavanone
Catalog No.:BCN1710
CAS No.:160436-10-2
- 30-Oxopseudotaraxasterol
Catalog No.:BCN7135
CAS No.:160481-71-0
- THZ1
Catalog No.:BCC4005
CAS No.:1604810-83-4
- THZ2
Catalog No.:BCC3986
CAS No.:1604810-84-5
- Antirhine
Catalog No.:BCN4003
CAS No.:16049-28-8
- Bisandrographolide A
Catalog No.:BCN4701
CAS No.:160498-00-0
AP-1 Inhibition by SR 11302 Protects Human Hepatoma HepG2 Cells from Bile Acid-Induced Cytotoxicity by Restoring the NOS-3 Expression.[Pubmed:27490694]
PLoS One. 2016 Aug 4;11(8):e0160525.
The harmful effects of bile acid accumulation occurring during cholestatic liver diseases have been associated with oxidative stress increase and endothelial nitric oxide synthase (NOS-3) expression decrease in liver cells. We have previously reported that glycochenodeoxycholic acid (GCDCA) down-regulates gene expression by increasing SP1 binding to the NOS-3 promoter in an oxidative stress dependent manner. In the present study, we aimed to investigate the role of transcription factor (TF) AP-1 on the NOS-3 deregulation during GCDCA-induced cholestasis. The cytotoxic response to GCDCA was characterized by 1) the increased expression and activation of TFs cJun and c-Fos; 2) a higher binding capability of these at position -666 of the NOS-3 promoter; 3) a decrease of the transcriptional activity of the promoter and the expression and activity of NOS-3; and 4) the expression increase of cyclin D1. Specific inhibition of AP-1 by the retinoid SR 11302 counteracted the cytotoxic effects induced by GCDCA while promoting NOS-3 expression recovery and cyclin D1 reduction. NOS activity inhibition by L-NAME inhibited the protective effect of SR 11302. Inducible NOS isoform was no detected in this experimental model of cholestasis. Our data provide direct evidence for the involvement of AP-1 in the NOS-3 expression regulation during cholestasis and define a critical role for NOS-3 in regulating the expression of cyclin D1 during the cell damage induced by bile acids. AP-1 appears as a potential therapeutic target in cholestatic liver diseases given its role as a transcriptional repressor of NOS-3.
Effects of novel RAR- and RXR-selective retinoids on myeloid leukemic proliferation and differentiation in vitro.[Pubmed:10068679]
Blood. 1999 Mar 15;93(6):2057-66.
Retinoids such as all-trans-retinoic acid (ATRA) and 9-cis-retinoic acid (9-cis-RA) have an important role in many aspects of proliferation and differentiation of hematopoietic cells. They exert their effects by binding to retinoic acid receptors (RARs) and/or retinoid X receptors (RXRs). We studied the effects of novel retinoids on proliferation and differentiation of HL-60 and NB4 myeloid leukemic cells, as well as acute promyelocytic leukemia (APL) cells from patients. RXR-selective SR11345 (Retinoid C) had little ability to inhibit the clonal growth and to induce the differentiation of either HL-60 or NB4 cells. However, SR11276 (Retinoid E), which activated both the RAR and RXR classes, and SR11278 (Retinoid D), which activated the RAR subtypes alpha, beta, and gamma, could inhibit clonal growth of both cell types, as well as leukemic cells from APL patients. The combination of ATRA and either SR11276 or SR11278 additively inhibited APL cell proliferation. SR11302 (Retinoid A), with reported anti-AP-1 activity and no activation of RARs and RXR and SR11363 (Retinoid B), which selectively activated RARbeta and gamma, were inactive. The clonal proliferation of both HL-60 and NB4 cells that were pulse-exposed to 10(-9) mol/L ATRA, SR11276, SR11278, or SR11345 for 3 days, washed, and plated in methylcellulose culture were inhibited by 0%, 51%, 21%, and 1% for HL-60 cells and 43%, 41%, 35%, and 1% for NB4, respectively, compared with nontreated control cells. When the HL-60 cells were pulse-exposed to 10(-9) mol/L of either SR11278 or SR11276, plus 10(-9) mol/L ATRA for 3 days, colony numbers were reduced by 46% and 64%, respectively. Induction of leukemic cell differentiation as determined by the nitroblue tetrazolium (NBT) assay showed that the combination of 10(-7) mol/L of either SR11278 or SR11276 with 10(-7) mol/L ATRA had additive effects on HL-60 cells, NB4 cells, and fresh APL cells. Induction of CD11b expression on both HL-60 and NB4 cells occurs during their differentiation. Expression of this antigen was synergistically augmented by the combination of either 10(-7) to 10(-8) mol/L SR11278 or 10(-7) to 10(-9) mol/L SR11276 with 10(-9) mol/L ATRA compared with either analog alone in HL-60 cells. Expression of the novel myeloid specific transcription factor C/EBPepsilon was increased by SR11278 and SR11276 in both the HL-60 and NB4 cell lines. We conclude that retinoids or combination of retinoids with specificities for both RAR and RXR may markedly enhance the ability of ATRA to inhibit clonal growth and induce differentiation of HL-60 and NB4 leukemic cells. This occurs in the absence of continuous contact with retinoids.
A new class of retinoids with selective inhibition of AP-1 inhibits proliferation.[Pubmed:7969403]
Nature. 1994 Nov 3;372(6501):107-11.
Retinoids regulate many biological processes, including differentiation, morphogenesis and cell proliferation. They are also important therapeutic agents, but their clinical usefulness is limited because of side effects. Retinoid activities are mediated by specific nuclear receptors, the RARs and RXRs, which can induce transcriptional activation through specific DNA sites or by inhibiting the transcription factor AP-1 (refs 12-15), which usually mediates cell proliferation signals. Because the two types of receptor actions are mechanistically distinct, we investigated whether conformationally restricted retinoids, selective for each type of receptor action, could be identified. Here we describe a new class of retinoids that selectively inhibits AP-1 activity but does not activate transcription. These retinoids do not induce differentiation in F9 cells but inhibit effectively the proliferation of several tumour cell lines, and could thus serve as candidates for new retinoid therapeutic agents with reduced side effects.


