SulfasalazineNF-κB activation inhibitor CAS# 599-79-1 |
- Rocilinostat (ACY-1215)
Catalog No.:BCC2144
CAS No.:1316214-52-4
- LY 294002
Catalog No.:BCC3659
CAS No.:154447-36-6
- Doxorubicin
Catalog No.:BCC2082
CAS No.:23214-92-8
- E 64d
Catalog No.:BCC1127
CAS No.:88321-09-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 599-79-1 | SDF | Download SDF |
| PubChem ID | 5339 | Appearance | Powder |
| Formula | C18H14N4O5S | M.Wt | 398.39 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | NSC 667219 | ||
| Solubility | DMSO : 80 mg/mL (200.81 mM; Need ultrasonic and warming) | ||
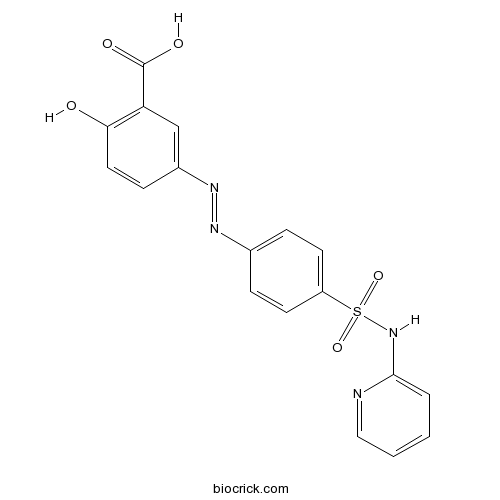
| Chemical Name | 2-hydroxy-5-[[4-(pyridin-2-ylsulfamoyl)phenyl]diazenyl]benzoic acid | ||
| SMILES | C1=CC=NC(=C1)NS(=O)(=O)C2=CC=C(C=C2)N=NC3=CC(=C(C=C3)O)C(=O)O | ||
| Standard InChIKey | NCEXYHBECQHGNR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H14N4O5S/c23-16-9-6-13(11-15(16)18(24)25)21-20-12-4-7-14(8-5-12)28(26,27)22-17-3-1-2-10-19-17/h1-11,23H,(H,19,22)(H,24,25) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of NF-κB activation. Inhibits in vitro growth of the human pancreatic cancer cell lines MIA PaCa-2 and PANC-1, and induces apoptosis in glioblastoma cell lines. Also inhibits the cystine-glutamate antiporter, system Xc (SXC). Anti-inflammatory. |

Sulfasalazine Dilution Calculator

Sulfasalazine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5101 mL | 12.5505 mL | 25.101 mL | 50.2021 mL | 62.7526 mL |
| 5 mM | 0.502 mL | 2.5101 mL | 5.0202 mL | 10.0404 mL | 12.5505 mL |
| 10 mM | 0.251 mL | 1.2551 mL | 2.5101 mL | 5.0202 mL | 6.2753 mL |
| 50 mM | 0.0502 mL | 0.251 mL | 0.502 mL | 1.004 mL | 1.2551 mL |
| 100 mM | 0.0251 mL | 0.1255 mL | 0.251 mL | 0.502 mL | 0.6275 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Inhibitor of NF-κB activation. Inhibits in vitro growth of the human pancreatic cell lines MIA PaCa-2 and PANC-1, and induces apoptosis in glioblastoma cell lines. Also inhibits the cystine-glutamate antiporter, system Xc (SXC). Anti-inflamm
- 3,3'-Sulfonyldianiline
Catalog No.:BCC8595
CAS No.:599-61-1
- Medicagenic acid
Catalog No.:BCN3893
CAS No.:599-07-5
- Mesopsin
Catalog No.:BCN8050
CAS No.:5989-16-2
- Loliolid
Catalog No.:BCN3655
CAS No.:5989-02-6
- 7-hydroxy-4-benzopyrone
Catalog No.:BCC9209
CAS No.:59887-89-7
- ent-7alpha,9-Dihydroxy-15-oxokaur-16-en-19,6bet-olide
Catalog No.:BCN7401
CAS No.:59885-89-1
- N-Formyl-Met-Leu-Phe
Catalog No.:BCC7205
CAS No.:59880-97-6
- L-Dihydroorotic acid
Catalog No.:BCC9007
CAS No.:5988-19-2
- Glabridin
Catalog No.:BCN1221
CAS No.:59870-68-7
- Oxybuprocaine HCl
Catalog No.:BCC4691
CAS No.:5987-82-6
- Cyclosporin A
Catalog No.:BCC4773
CAS No.:59865-13-3
- Patchouli alcohol
Catalog No.:BCN4966
CAS No.:5986-55-0
- 3-Hydroxy-8,9-methylenedioxypterocarpene
Catalog No.:BCN1407
CAS No.:59901-98-3
- Vicenin -3
Catalog No.:BCN3014
CAS No.:59914-91-9
- HPI 1
Catalog No.:BCC3938
CAS No.:599150-20-6
- Vindesine sulfate
Catalog No.:BCC8266
CAS No.:59917-39-4
- Vicriviroc maleate
Catalog No.:BCC2038
CAS No.:599179-03-0
- 3',4'-dihydro-3'-hydroxy-Xanthyletin
Catalog No.:BCN3680
CAS No.:5993-18-0
- Malotilate
Catalog No.:BCC1196
CAS No.:59937-28-9
- Carlinoside
Catalog No.:BCN2853
CAS No.:59952-97-5
- Tagitinin F
Catalog No.:BCN4101
CAS No.:59979-57-6
- Tagitinin A
Catalog No.:BCN4102
CAS No.:59979-61-2
- EDTA
Catalog No.:BCC7493
CAS No.:60-00-4
- Guanethidine Sulfate
Catalog No.:BCC3789
CAS No.:60-02-6
Comparative Effectiveness of Mesalamine, Sulfasalazine, Corticosteroids, and Budesonide for the Induction of Remission in Crohn's Disease: A Bayesian Network Meta-analysis.[Pubmed:28146003]
Inflamm Bowel Dis. 2017 Mar;23(3):461-472.
BACKGROUND: Induction treatment of mild-to-moderate Crohn's disease is controversial. PURPOSE: To compare the induction of remission between different doses of mesalamine, Sulfasalazine, corticosteroids, and budesonide for active Crohn's disease. DATA SOURCES: We identified randomized controlled trials from existing Cochrane reviews and an updated literature search in Medline, EMBASE, and CENTRAL to November 2015. STUDY SELECTION: We included randomized controlled trials (n = 22) in adult patients with Crohn's disease that compared budesonide, Sulfasalazine, mesalamine, or corticosteroids with placebo or each other, for the induction of remission (8-17 wks). Mesalamine (above and below 2.4 g/d) and budesonide (above and below 6 mg/d) were stratified into low and high doses. DATA EXTRACTION: Our primary outcome was remission, defined as a Crohn's Disease Activity Index score <150. A Bayesian random-effects network meta-analysis was performed on the proportion in remission. DATA SYNTHESIS: Corticosteroids (odds ratio [OR] = 3.80; 95% credible interval [CrI]: 2.48-5.66), high-dose budesonide (OR = 2.96; 95% CrI: 2.06-4.30), and high-dose mesalamine (OR = 2.29; 95% CrI: 1.58-3.33) were superior to placebo. Corticosteroids were similar to high-dose budesonide (OR = 1.21; 95% CrI: 0.84-1.76), but more effective than high-dose mesalamine (OR = 1.83; 95% CrI: 1.16-2.88). Sulfasalazine was not significantly superior to any therapy including placebo. LIMITATIONS: Randomized controlled trials that use a strict definition of induction of remission and disease severity at enrollment to assess effectiveness in treating mild-to-moderate Crohn's disease are limited. CONCLUSIONS: Corticosteroids and high-dose budesonide were effective treatments for inducing remission in mild-to-moderate Crohn's disease. High-dose mesalamine is an option among patients preferring to avoid steroids.
Real-life experience of using conventional disease-modifying anti-rheumatic drugs (DMARDs) in psoriatic arthritis (PsA). Retrospective analysis of the efficacy of methotrexate, sulfasalazine, and leflunomide in PsA in comparison to spondyloarthritides other than PsA and literature review of the use of conventional DMARDs in PsA.[Pubmed:28293446]
Eur J Rheumatol. 2017 Mar;4(1):1-10.
OBJECTIVE: With the aim of assessing the response to treatment with conventional disease-modifying anti-rheumatic drugs (DMARDs) used in patients with psoriatic arthritis (PsA), data on methotrexate, Sulfasalazine (SSZ), and leflunomide were analyzed from baseline and subsequent follow-up (FU) questionnaires completed by patients with either PsA or other spondyloarthritides (SpAs). MATERIAL AND METHODS: A single-center real-life retrospective analysis was performed by obtaining clinical data via questionnaires administered before and after treatment. The indices used were erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) level, Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Bath Ankylosing Spondylitis Function Index (BASFI), wellbeing (WB), and treatment effect (TxE). The indices measured at baseline were compared with those measured on one occasion in a FU visit at least 1 year later. RESULTS: A total of 73 patients, 51 with PsA (mean age 49.8+/-12.8 years; male-to-female ratio [M:F]=18:33) and 22 with other SpAs (mean age 50.6+/-16 years; M:F=2:20), were studied. BASDAI, BASFI, and WB displayed consistent improvements during FU assessments in both PsA patients and controls in comparison to baseline values. SSZ exhibited better efficacy as confirmed by TxE in both PsA patients and controls. ESR and CRP displayed no differences in either the PsA or the SpA group between the cases before and after treatment. CONCLUSION: Real-life retrospective analysis of three DMARDs used in PsA (and SpAs other than PsA) demonstrated that all three DMARDs that were used brought about improvements in BASDAI, BASFI, TxE, and WB. However, the greatest improvements at FU were seen with SSZ use in both PsA and control cohorts.
Sulfasalazine attenuates evading anticancer response of CD133-positive hepatocellular carcinoma cells.[Pubmed:28253902]
J Exp Clin Cancer Res. 2017 Mar 3;36(1):38.
BACKGROUND: CD133-positive cells in hepatocellular carcinoma (HCC) exhibit cancer stem cell (CSC)-like properties as well as resistance to chemotherapeutic agents and ionizing radiation; however, their function remains unknown. In this paper, we identified a hitherto unknown mechanism to overcome CD133-induced resistance to anticancer therapy. METHODS: We applied an alternative approach to enrich the CD133-positive HCC population by manipulating 3D culture conditions. Defense mechanisms against reactive oxygen species (ROS) in CSC spheroids were evaluated by fluorescence image-based phenotypic screening system. Further, we studied the effect of Sulfasalazine on ROS defense system and synergistic therapeutic efficacy of anticancer therapies both in culture and in vivo HCC xenograft mouse model. RESULTS: Here, we found that oxidative stress increase CD133 expression in HCC and increased CD133 expression enhanced the capacity of the defense system against ROS, and thereby play a central role in resistance to liver cancer therapy. Moreover, ablation of CD133 attenuated not only the capacity for defense against ROS, but also chemoresistance, in HCC through decreasing glutathione (GSH) levels in vitro. Sulfasalazine, a potent xCT inhibitor that plays an important role in maintaining GSH levels, impaired the ROS defense system and increased the therapeutic efficacy of anticancer therapies in CD133-positive HCC but not CD133-negative HCC in vivo and in vitro. CONCLUSION: These results strongly indicate functional roles for CD133 in ROS defense and in evading anticancer therapies in HCC, and suggest that Sulfasalazine, administered in combination with conventional chemotherapy, might be an effective strategy against CD133-positive HCC cells.
Sulfasalazine for brain cancer fits.[Pubmed:22404218]
Expert Opin Investig Drugs. 2012 May;21(5):575-8.
Recent research has identified an important role for a cystine-glutamate antiporter (system Xc) in the biology of malignant brain tumors. This transporter is effectively inhibited by Sulfasalazine, a drug widely used to treat a number of chronic inflammatory conditions such as Crohn's disease. Preclinical data show that Sulfasalazine is an effective inhibitor of tumor growth and tumor-associated seizures. These studies suggest that the cystine-glutamate antiporter is a valuable drug target for which tumor-specific drugs can be generated. In the meantime, Sulfasalazine may be considered as adjuvant treatment for malignant gliomas.
Potential use of the anti-inflammatory drug, sulfasalazine, for targeted therapy of pancreatic cancer.[Pubmed:20567622]
Curr Oncol. 2010 Jun;17(3):9-16.
Pancreatic cancer is an aggressive, drug-resistant disease; its first-line chemotherapeutic, gemcitabine, is only marginally effective. Intracellular depletion of glutathione, a major free-radical scavenger, has been associated with growth arrest and reduced drug resistance (chemosensitization) of cancer cells. In search of a new therapeutic approach for pancreatic cancer, we sought to determine whether specific inhibition of the plasma membrane x(c) (-) cystine transporter could lead to reduced uptake of cysteine, a key precursor of glutathione, and subsequent glutathione depletion. Sulfasalazine (approximately 0.2 mmol/L), an anti-inflammatory drug with potent x(c) (-)-inhibitory properties, markedly reduced l(1)(4)C]-cystine uptake, glutathione levels, and growth and viability of human MIA PaCa-2 and PANC-1 pancreatic cancer cells in vitro. These effects were shown to result primarily from inhibition of cystine uptake mediated by the x(c) (-) cystine transporter and not from inhibition of nuclear factor kappaB activation, another property of Sulfasalazine. The efficacy of gemcitabine could be markedly enhanced by combination therapy with Sulfasalazine both in vitro and in immunodeficient mice carrying xenografts of the same cell lines. No major side effects were observed in vivo.The results of the present study suggest that the x(c) (-) transporter plays a major role in pancreatic cancer by sustaining or enhancing glutathione biosynthesis, and as such, represents a potential therapeutic target. Sulfasalazine, a relatively nontoxic drug approved by the U.S. Food and Drug Administration, may, in combination with gemcitabine, lead to more effective therapy of refractory pancreatic cancer.


