Vicriviroc maleateCCR5 antagonist CAS# 599179-03-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 599179-03-0 | SDF | Download SDF |
| PubChem ID | 6451165 | Appearance | Powder |
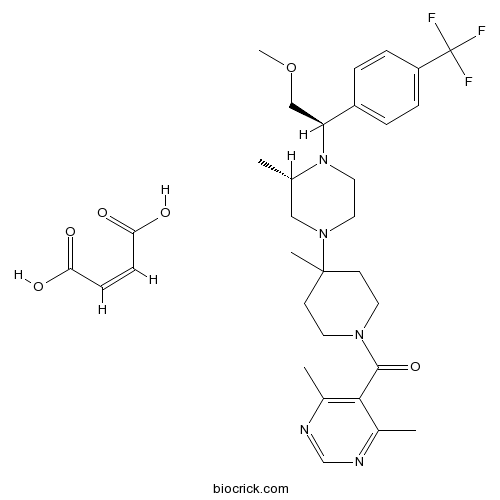
| Formula | C32H42F3N5O6 | M.Wt | 649.7 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | SCH-417690 (maleate); SCH-D (maleate) | ||
| Solubility | DMSO : 50 mg/mL (76.96 mM; Need ultrasonic) H2O : 25 mg/mL (38.48 mM; Need ultrasonic and warming) | ||
| Chemical Name | (Z)-but-2-enedioic acid;(4,6-dimethylpyrimidin-5-yl)-[4-[(3S)-4-[(1R)-2-methoxy-1-[4-(trifluoromethyl)phenyl]ethyl]-3-methylpiperazin-1-yl]-4-methylpiperidin-1-yl]methanone | ||
| SMILES | CC1CN(CCN1C(COC)C2=CC=C(C=C2)C(F)(F)F)C3(CCN(CC3)C(=O)C4=C(N=CN=C4C)C)C.C(=CC(=O)O)C(=O)O | ||
| Standard InChIKey | GXINKQQWHLIBJA-UCIBKFKQSA-N | ||
| Standard InChI | InChI=1S/C28H38F3N5O2.C4H4O4/c1-19-16-35(14-15-36(19)24(17-38-5)22-6-8-23(9-7-22)28(29,30)31)27(4)10-12-34(13-11-27)26(37)25-20(2)32-18-33-21(25)3;5-3(6)1-2-4(7)8/h6-9,18-19,24H,10-17H2,1-5H3;1-2H,(H,5,6)(H,7,8)/b;2-1-/t19-,24-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Vicriviroc maleate is a potent, selective, oral bioavailable and CNS penetrated antagonist of CCR5, with a Ki of 2.5 nM, and also inhibits HIV-1 in PBMC cells, with IC90s of 3.3 nM (JrFL), 2.8 nM (ADA-M), 1.8 nM (301657), 4.9 nM (JV1083) and 10 nM (RU 570).In Vitro:Vicriviroc is a potent, selective and oral bioavailable inhibitor of CCR5, with a Ki of 2.5 nM, and also inhibits HIV-1 in PBMC cells, with IC90s of 3.3 (JrFL), 2.8 (ADA-M), 1.8 (301657), 4.9 (JV1083) and 10 nM (RU 570). In addition, Vicriviroc shows a mean IC50 and IC90 of 0.45 nM and 4 nM for a panel of HIV isolates, and has weak activity against hERG activity (IC50, 5.8 μM)[1]. Vicriviroc inhibits chemotactic response to MIP-1α with IC50 values below 1 nM, and suppresses RANTES-induced signaling with a mean IC50 of 4.2 ± 1.3 nM. Vicriviroc potently suppresses all the viral isolates tested, with geometric mean EC50s of 0.04-2.3 nM and IC90s of 0.45-18 nM[2].In Vivo:Vicriviroc (10 mg/kg) has good oral availablity in rats and monkeys, with no acute CNS or GI effects in rats[1]. References: | |||||

Vicriviroc maleate Dilution Calculator

Vicriviroc maleate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5392 mL | 7.6959 mL | 15.3917 mL | 30.7834 mL | 38.4793 mL |
| 5 mM | 0.3078 mL | 1.5392 mL | 3.0783 mL | 6.1567 mL | 7.6959 mL |
| 10 mM | 0.1539 mL | 0.7696 mL | 1.5392 mL | 3.0783 mL | 3.8479 mL |
| 50 mM | 0.0308 mL | 0.1539 mL | 0.3078 mL | 0.6157 mL | 0.7696 mL |
| 100 mM | 0.0154 mL | 0.077 mL | 0.1539 mL | 0.3078 mL | 0.3848 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Vicriviroc is a CCR5 antagonist with IC50 of 0.91 nM in clinical development for the treatment of HIV-1. Two phase I studies were conducted to assess the safety of Vicriviroc. Vicriviroc maleate is currently in late-stage clinical development as part of a ritonavir-boosted protease inhibitor regimen for HIV-1-infected individuals. In clinical studies, Vicriviroc has demonstrated potent and durable virologic suppression, immunologic activity, and generally favorable tolerability.
- Vindesine sulfate
Catalog No.:BCC8266
CAS No.:59917-39-4
- HPI 1
Catalog No.:BCC3938
CAS No.:599150-20-6
- Vicenin -3
Catalog No.:BCN3014
CAS No.:59914-91-9
- 3-Hydroxy-8,9-methylenedioxypterocarpene
Catalog No.:BCN1407
CAS No.:59901-98-3
- Sulfasalazine
Catalog No.:BCC2545
CAS No.:599-79-1
- 3,3'-Sulfonyldianiline
Catalog No.:BCC8595
CAS No.:599-61-1
- Medicagenic acid
Catalog No.:BCN3893
CAS No.:599-07-5
- Mesopsin
Catalog No.:BCN8050
CAS No.:5989-16-2
- Loliolid
Catalog No.:BCN3655
CAS No.:5989-02-6
- 7-hydroxy-4-benzopyrone
Catalog No.:BCC9209
CAS No.:59887-89-7
- ent-7alpha,9-Dihydroxy-15-oxokaur-16-en-19,6bet-olide
Catalog No.:BCN7401
CAS No.:59885-89-1
- N-Formyl-Met-Leu-Phe
Catalog No.:BCC7205
CAS No.:59880-97-6
- 3',4'-dihydro-3'-hydroxy-Xanthyletin
Catalog No.:BCN3680
CAS No.:5993-18-0
- Malotilate
Catalog No.:BCC1196
CAS No.:59937-28-9
- Carlinoside
Catalog No.:BCN2853
CAS No.:59952-97-5
- Tagitinin F
Catalog No.:BCN4101
CAS No.:59979-57-6
- Tagitinin A
Catalog No.:BCN4102
CAS No.:59979-61-2
- EDTA
Catalog No.:BCC7493
CAS No.:60-00-4
- Guanethidine Sulfate
Catalog No.:BCC3789
CAS No.:60-02-6
- H-Tyr-OH
Catalog No.:BCC3123
CAS No.:60-18-4
- Acetylcholine chloride
Catalog No.:BCN2197
CAS No.:60-31-1
- (6-)ε-Aminocaproic acid
Catalog No.:BCC4888
CAS No.:60-32-2
- Linoleic acid
Catalog No.:BCN3821
CAS No.:60-33-3
- Acetamide
Catalog No.:BCN4114
CAS No.:60-35-5
Gateways to clinical trials.[Pubmed:21225012]
Methods Find Exp Clin Pharmacol. 2010 Dec;32(10):749-73.
Gateways to Clinical Trials is a guide to the most recent clinical trials in current literature and congresses. The data in the following tables has been retrieved from the Clinical Trials Knowledge Area of Thomson Reuters Integrity(SM), the drug discovery and development portal, http://www.thomsonreutersintegrity.com. This issue focuses on the following selection of drugs: 17-Hydroxyprogesterone caproate; Abacavir sulfate/lamivudine, Aclidinium bromide, Adalimumab, Adefovir, Alemtuzumab, Alkaline phosphatase, Amlodipine, Apilimod mesylate, Aripiprazole, Axitinib, Azacitidine; Belotecan hydrochloride, Berberine iodide, Bevacizumab, Bortezomib, Bosentan, Bryostatin 1; Calcipotriol/hydrocortisone, Carglumic acid, Certolizumab pegol, Cetuximab, Cinacalcet hydrochloride, Cixutumumab, Coumarin, Custirsen sodium; Darbepoetin alfa, Darifenacin hydrobromide, Darunavir, Dasatinib, Denibulin hydrochloride, Denosumab, Diacetylmorphine, Dulanermin, Duloxetine hydrochloride; Ecogramostim, Enfuvirtide, Entecavir, Enzastaurin hydrochloride, Eplerenone, Escitalopram oxalate, Esomeprazole sodium, Etravirine, Everolimus, Ezetimibe; Fenofibrate/pravastatin sodium, Ferric carboxymaltose, Flavangenol, Fondaparinux sodium; Glutamine, GSK-1024850A; Hepatitis B hyperimmunoglobulin, Hib-MenC, HIV-LIPO-5; Immunoglobulin intravenous (human), Indacaterol maleate, Indibulin, Indium 111 ((1)(1)(1)In) ibritumomab tiuxetan, Influenza A (H1N1) 2009 Monovalent vaccine, Inhalable human insulin, Insulin glulisine; Lapatinib ditosylate, Leucovorin/UFT; Maraviroc, Mecasermin, MMR-V, Morphine hydrochloride, Morphine sulfate/naltrexone hydrochloride, Mycophenolic acid sodium salt; Naproxen/esomeprazole magnesium, Natalizumab; Oncolytic HSV; Paliperidone, PAN-811, Paroxetine, Pegfilgrastim, Peginterferon alfa-2a, Peginterferon alfa-2b/ribavirin, Pegvisomant, Pemetrexed disodium, Pimecrolimus, Posaconazole, Pregabalin; Raltegravir potassium, Ranelic acid distrontium salt, Rasburicase, Rilpivirine hydrochloride; Sertindole, Sivelestat sodium hydrate, Sorafenib, Sumatriptan succinate/naproxen sodium, Sunitinib malate; Tafluprost, Telithromycin, Temsirolimus, Tenofovir disoproxil fumavate, Tenofovir disoproxil fumarate/emtricitabine, Teriparatide, Ticagrelor, Tigecycline, Tipranavir, Tirapazamine, Trimetrexate; Ulipristal acetate; Valganciclovir hydrochloride, Vicriviroc, Vorinostat; Yttrium 90 (90Y) ibritumomab tiuxetan.
Gateways to clinical trials.[Pubmed:19907722]
Methods Find Exp Clin Pharmacol. 2009 Sep;31(7):463-93.
Gateways to Clinical Trials is a guide to the most recent clinical trials in current literature and congresses. The data in the following tables has been retrieved from the Clinical Trials Knowledge Area of Prous Science Integrity, the drug discovery and development portal, http://integrity.prous.com. This issue focuses on the following selection of drugs: AAV1/SERCA2a, Abacavir sulfate/lamivudine, Adalimumab, Aliskiren fumarate, Ambrisentan, Aripiprazole, AT-7519, Atazanavir sulfate, Atomoxetine hydrochloride, Azacitidine, Azelnidipine; Besifloxacin hydrochloride, Bevacizumab, Bioabsorbable everolimus-eluting coronary stent, Bortezomib, Bosentan, Budesonide/formoterol fumarate; CAIV-T, Carisbamate, Casopitant mesylate, Certolizumab pegol, Cetuximab, Ciclesonide, Ciprofloxacin/dexamethasone, CTCE-9908; Dalcetrapib, Darunavir, Deferasirox, Desloratadine, Disitertide, Drotrecogin alfa (activated), DTA-H19, Duloxetine hydrochloride, Dutasteride; Ecogramostim, Efalizumab, Emtricitabine, Eribulin mesilate, Escitalopram oxalate, Eszopiclone, EUR-1008, Everolimus-eluting coronary stent, Exenatide; Fampridine, Fluticasone furoate, Formoterol fumarate/fluticasone propionate, Fosamprenavir calcium, Fulvestrant; Gabapentin enacarbil, GS-7904L; HPV-6/11/16/18, Human Secretin, Hydralazine hydrochloride/isosorbide dinitrate; Imatinib mesylate, Imexon, Inalimarev/Falimarev, Indacaterol, Indacaterol maleate, Inhalable human insulin, Insulin detemir, Insulin glargine, Ixabepilone; L-Alanosine, Lapatinib ditosylate, Lenalidomide, Levocetirizine dihydrochloride, Liraglutide, Lisdexamfetamine mesilate, Lopinavir, Loratadine/montelukast sodium, Lutropin alfa; MeNZB, Mepolizumab, Micafungin sodium, Morphine hydrochloride; Nabiximols, Nikkomycin Z; Olmesartan medoxomil, Omalizumab; Paclitaxel-eluting stent, Pegfilgrastim, Peginterferon alfa-2a, Peginterferon alfa-2b, Perifosine, PF-489791, Plitidepsin, Posaconazole, Pregabalin; QAX-576; Raltegravir potassium, Ramelteon, Rasagiline mesilate, Recombinant human relaxin H2, rhGAD65, Rivaroxaban, Rosuvastatin calcium, Rotigotine; Saxagliptin, SCH-530348, Sirolimus-eluting stent, SLIT-amikacin, Sorafenib, Sotrastaurin, SR-16234, Sulforaphane; Tadalafil, Tanespimycin, Tapentadol hydrochloride, Teriparatide, Tesofensine, Tiotropium bromide, Tipifarnib, Tirapazamine, TMC-207, Tocilizumab, Tolvaptan, Tosedostat, Treprostinil sodium; Ustekinumab; Varespladib methyl, Vicriviroc, Vildagliptin, Vildagliptin/metformin hydrochloride, Volociximab, Voriconazole; Ziconotide, Ziprasidone hydrochloride.
Gateways to clinical trials.[Pubmed:20664824]
Methods Find Exp Clin Pharmacol. 2010 Jun;32(5):331-88.
[(1)(1)C]RAC; (18)F-Fluoromisonidazole; 89-12; 9-[(1)(8)F]Fluoropropyl-(+)-dihydrotetrabenazine; Adalimumab, Adecatumumab, ADMVA, ADXS-11-001, Aflibercept, Agatolimod sodium, AGS-004, Alglucosidase alfa, Aliskiren fumarate, Alvocidib hydrochloride, AMG-108, AMG-853, Apixaban, Aripiprazole, Armodafinil, Atazanavir sulfate, Atomoxetine hydrochloride; Bevacizumab, BioMatrix Flex drug eluting stent, Biphasic insulin aspart, Bortezomib, Bosentan; Caspofungin acetate, Cediranib, Cetuximab, ChimeriVax-Dengue, Choriogonadotropin alfa, Cinacalcet hydrochloride, Cizolirtine citrate, Clofarabine, Cocaine conjugate vaccine, CX-717; Darbepoetin alfa, Dasatinib, Decitabine, Denosumab, Desvenlafaxine succinate, Dexamethasone sodium phosphate, Dienogest, Diphencyprone, Doripenem, DTaP-HepB-IPV, Dutasteride; E-7010, Ecallantide, Ecstasy, Eicosapentaenoic acid/docosahexaenoic acid, Emtricitabine, Enfuvirtide, Erlotinib hydrochloride, Eszopiclone, Etonogestrel/ethinyl estradiol, Etoricoxib, Everolimus, Everolimus-eluting coronary stent EVT-201, Ezetimibe, Ezetimibe/simvastatin; Ferumoxytol, Fesoterodine fumavate, Figitumumab, Filgrastim, Fingolimod hydrochloride, Fluticasone furoate, Fluval P, Fluzone, Fondaparinux sodium, Fulvestrant, Fungichromin; Gamma-hydroxybutyrate sodium, Gefitinib, GHB-01L1, GLY-230, GSK-1349572; Hib-MenCY-TT, Hib-TT, HPV-6/11/16/18, Hydrocodone bitartrate; IC-51, Icatibant acetate, Imatinib mesylate, Immunoglobulin intravenous (human), Indetanib, Influenza A (H1N1) 2009 Monovalent Vaccine, Inhalable human insulin, Insulin glargine, Insulin glulisine, Interferon-beta, Ispinesib mesylate, Ixabepilone; Laromustine, Latanoprost/timolol maleate, L-Citrulline, Lenalidomide, Lexatumumab, Linezolid, Lopinavir/ritonavir, Lutropin alfa; Mapatumumab, MDX-066, MDX-1388, Mepolizumab, Methoxy polyethylene glycol-epoetin-beta, Metreleptin, Micafungin sodium, Mometasone furoate/oxymetazoline hydrochloride, Mx-dnG1, Mycophenolic acid sodium salt; Nabiximols, Natalizumab, Nemonoxacin, Norelgestromin/ethinyl estradiol; Oblimersen sodium, Ocriplasmin, Olmesartan medoxomil, Omacetaxine mepesuccinate; Paclitaxel-eluting stent, Pagoclone, Paliperidone, Panitumumab, Pazopanib hydrochloride, PCV7, Pegaptanib octasodium, Peginterferon alfa-2a, Peginterferon alfa-2b/ ribavirin, Pegvisomant, Pemetrexed disodium, Perifosine, Pimecrolimus, Pitavastatin calcium, Plerixafor hydrochloride, Plitidepsin, Posaconazole, Pregabalin, Progesterone capriate; Raltegravir potassium, Ramucirumab, Ranelic acid distrontium salt, Rasburicase, Recombinant Bet V1, Recombinant human insulin, rhFSH, Rolofylline, Romidepsin, Romiplostim, Rosuvastatin calcium; Sapacitabine, Sevelamer carbonate, Sinecatechins, Sirolimus-eluting stent, Sitagliptin phosphate monohydrate, SN-29244, Sorafenib, Sugammadex sodium, Sunitinib malate; Tadalafil, Tafenoquine, Talnetant, Tanezumab, Tapentadol hydrochloride, Tasocitinib citrate, Technosphere/Insulin, Telcagepant, Tenofovir disoproxil fumarate, Teriparatide, Ticagrelor, Tigecycline, Tiotropium bromide, Tipifarnib, Tocilizumab, TS-041; Ulipristal acetate, Urtoxazumab, Ustekinumab; Vandetanib, Varenicline tartrate, Vicriviroc, Voriconazole, Vorinostat, VRC-HIVADV014-00-VP, VRC-HIVDNA016-00-VP; Zoledronic acid monohydrate.


