N-Formyl-Met-Leu-PheEndogenous FPR1 agonist CAS# 59880-97-6 |
- SU14813
Catalog No.:BCC1971
CAS No.:627908-92-3
Quality Control & MSDS
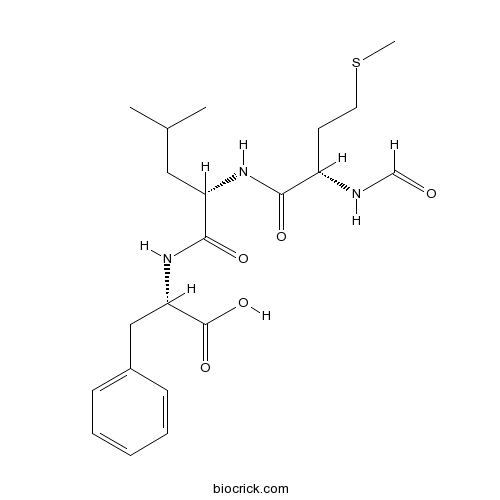
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 59880-97-6 | SDF | Download SDF |
| PubChem ID | 443295 | Appearance | Powder |
| Formula | C21H31N3O5S | M.Wt | 437.55 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | fMLP | ||
| Solubility | Soluble to 4.38 mg/ml in DMSO | ||
| Sequence | MLF (Modifications: Met-1 = N-formyl-Met) | ||
| Chemical Name | (2S)-2-[[(2S)-2-[[(2S)-2-formamido-4-methylsulfanylbutanoyl]amino]-4-methylpentanoyl]amino]-3-phenylpropanoic acid | ||
| SMILES | CC(C)CC(C(=O)NC(CC1=CC=CC=C1)C(=O)O)NC(=O)C(CCSC)NC=O | ||
| Standard InChIKey | PRQROPMIIGLWRP-BZSNNMDCSA-N | ||
| Standard InChI | InChI=1S/C21H31N3O5S/c1-14(2)11-17(23-19(26)16(22-13-25)9-10-30-3)20(27)24-18(21(28)29)12-15-7-5-4-6-8-15/h4-8,13-14,16-18H,9-12H2,1-3H3,(H,22,25)(H,23,26)(H,24,27)(H,28,29)/t16-,17-,18-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Endogenous chemotactic peptide and agonist for the formyl peptide receptor 1 (FPR1) (Ki = 38 nM). Stimulates aggregation of leukocytes. |

N-Formyl-Met-Leu-Phe Dilution Calculator

N-Formyl-Met-Leu-Phe Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
N-Formyl-Met-Leu-Phe (fMLP; N-Formyl-MLF) i a chemotactic peptide and a pecific ligand of N-formyl peptide receptor (FPR). N-Formyl-Met-Leu-Ph i reported to inhibit TNF-alpha ecretion.
In Vitro:Binding of N-Formyl-Met-Leu-Phe to its specific cell surface receptor, N-formyl peptide receptor (FPR), triggers different cascades of biochemical events, eventually leading to cellular activation. FPR is a chemoattractant receptor belonging to the G protein-coupled receptor family. N-Formyl-Met-Leu-Phe promotes osteoblastic commitment and suppresses adipogenic commitment under osteoblastic differentiation conditions. N-Formyl-Met-Leu-Phe stimulates osteogenesis is associated with increased expression of osteogenic markers and mineralization. N-Formyl-Met-Leu-Phe inhibits expression of peroxisome proliferator-activated receptor-γ1. N-Formyl-Met-Leu-Phe-stimulated osteogenic differentiation is mediated via FPR1-phospholipase C/phospholipase D-Ca2+-calmodulin-dependent kinase II-ERK-CREB signaling pathways[1]. N-Formyl-Met-Leu-Phe, a bacterial-derived peptide, induced proinflammatory cytokine gene expression in human peripheral blood monocytes. Bacterial products LPS and N-Formyl-Met-Leu-Phe synergistically induce inflammatory response via multiple signaling pathways. TLR4, IKKβ-IκBα, and NF-κB signaling pathways are involved in the synergistic induction of TNF-α via p65 nuclear translocation-dependent mechanisms[2].
In Vivo:N-Formyl-Met-Leu-Phe promotes bone formation in zebrafish and rabbits. Extensive skeletal development is evident at 5 dpf in over 80% of N-Formyl-Met-Leu-Phe-treated zebrafish. Treatment with N-Formyl-Met-Leu-Phe results in increased expression of Runx2. Bone marrow spaces are widely formed, and connective tissue covering bone is dense, like periosteum, in N-Formyl-Met-Leu-Phe-treated calvaria[1]. N-Formyl-Met-Leu-Phe mediate release of calprotectin from PMN in vitro. It induces release of calprotectin from PMN in a dose dependent manner. A minimum of 10% of total PMN calprotectin is retained at concentrations of 0.1-10.0 nM of N-Formyl-Met-Leu-Phe[3].
References:
[1]. Shin MK, et al. N-formyl-methionyl-leucyl-phenylalanine (fMLP) promotes osteoblast differentiation via the N-formyl peptide receptor 1-mediated signaling pathway in human mesenchymal stem cells from bone marrow. J Biol Chem. 2011 May 13;286(19):17133-43.
[2]. Chen LY, et al. Synergistic induction of inflammation by bacterial products lipopolysaccharide and fMLP: an important microbial pathogenic mechanism. J Immunol. 2009 Feb 15;182(4):2518-24.
[3]. Hetland G, et al. Chemotaxins C5a and fMLP induce release of calprotectin (leucocyte L1 protein) from polymorphonuclear cells in vitro. Mol Pathol. 1998 Jun;51(3):143-8.
- L-Dihydroorotic acid
Catalog No.:BCC9007
CAS No.:5988-19-2
- Glabridin
Catalog No.:BCN1221
CAS No.:59870-68-7
- Oxybuprocaine HCl
Catalog No.:BCC4691
CAS No.:5987-82-6
- Cyclosporin A
Catalog No.:BCC4773
CAS No.:59865-13-3
- Patchouli alcohol
Catalog No.:BCN4966
CAS No.:5986-55-0
- Palustrol
Catalog No.:BCN4100
CAS No.:5986-49-2
- Synephrine HCl
Catalog No.:BCC4359
CAS No.:5985-28-4
- Ajmalimine
Catalog No.:BCN3420
CAS No.:59846-31-0
- Tenoxicam
Catalog No.:BCC4733
CAS No.:59804-37-4
- UK 14,304
Catalog No.:BCC5226
CAS No.:59803-98-4
- Vindolinine
Catalog No.:BCN7822
CAS No.:5980-02-9
- Cyclosporin C
Catalog No.:BCC8160
CAS No.:59787-61-0
- ent-7alpha,9-Dihydroxy-15-oxokaur-16-en-19,6bet-olide
Catalog No.:BCN7401
CAS No.:59885-89-1
- 7-hydroxy-4-benzopyrone
Catalog No.:BCC9209
CAS No.:59887-89-7
- Loliolid
Catalog No.:BCN3655
CAS No.:5989-02-6
- Mesopsin
Catalog No.:BCN8050
CAS No.:5989-16-2
- Medicagenic acid
Catalog No.:BCN3893
CAS No.:599-07-5
- 3,3'-Sulfonyldianiline
Catalog No.:BCC8595
CAS No.:599-61-1
- Sulfasalazine
Catalog No.:BCC2545
CAS No.:599-79-1
- 3-Hydroxy-8,9-methylenedioxypterocarpene
Catalog No.:BCN1407
CAS No.:59901-98-3
- Vicenin -3
Catalog No.:BCN3014
CAS No.:59914-91-9
- HPI 1
Catalog No.:BCC3938
CAS No.:599150-20-6
- Vindesine sulfate
Catalog No.:BCC8266
CAS No.:59917-39-4
- Vicriviroc maleate
Catalog No.:BCC2038
CAS No.:599179-03-0
A practical synthesis of the 16C/15N-labelled tripeptide N-formyl-Met-Leu-Phe, useful as a reference in solid-state NMR spectroscopy.[Pubmed:18982075]
Beilstein J Org Chem. 2008;4:35.
A mild synthetic method for N-Formyl-Met-Leu-Phe-OH (1) is described. After Fmoc solid phase peptide synthesis, on-bead formylation and HPLC purification, more than 30 mg of the fully (13)C/(15)N-labelled tripeptide 1 could be isolated in a typical batch. This peptide can be easily crystallised and is therefore well suited as a standard sample for setting up solid-state NMR experiments.
Guinea pig ileum motility stimulation elicited by N-formyl-Met-Leu-Phe (fMLF) involves neurotransmitters and prostanoids.[Pubmed:21126546]
Peptides. 2011 Feb;32(2):266-71.
In guinea-pig ileum (GPI), the chemotactic peptide N-Formyl-Met-Leu-Phe-OH (fMLF) possesses spasmogenic properties through the activation of formyl peptide receptors (FPRs). Despite this, the mediators involved remain to be elucidated. fMLF (1nM-1muM) induced a dose-dependent contraction of GPI (EC(50)=24nM), that is blocked by pre-treatment with the FPRs antagonist Boc(2). The pre-treatment with tetrodotoxin (TTX) atropine or with SR140333 reduced the fMLF-induced contraction, whereas with hexamethonium, MEN10627, SB222200, mepyramine, cimetidine, thioperamide or methysergide did not produce any effect. With DuP697 pre-treatment, but not with piroxicam, reduced the fMLF-induced contraction. After stimulation with 24nM fMLF, a strong increase in the PGE(2) levels was observed. Finally, the concomitant blocking of the NK(1) receptor, the muscarinic receptors and COX-2 abolished the GPI contractions induced by fMLF. fMLF induced a concentration-dependent contraction of guinea-pig jejunum (EC(50)=11nM), proximal colon (EC(50)=3.5nM) and distal colon (EC(50)=2.2nM), with a time-course similar to that observed in GPI. In these preparations as well, the co-administration of atropine, SR140333 and DuP697 abolished the contractions induced by fMLF. Intraperitoneal injection of fMLF (0.1 or 1mumol/kg) enhanced the gastrointestinal motility in mice, abolished by the co-administration of atropine, SR140333 and DuP697. In conclusion, we showed that fMLF exerts spasmogenic actions on guinea-pig intestine both in vitro and in vivo through the release of acetylcholine and substance P from myenteric motorneurons and through prostanoids, probably from the inflammatory cells of the enteric immune system.
Temperature pretreatment alters the polarization response of human neutrophils to the chemoattractant N-formyl-Met-Leu-Phe.[Pubmed:19067145]
Inflammation. 2009 Feb;32(1):47-56.
Neutrophils present a polarized morphology upon stimulation of chemoattractants, which play a vital role in host-defense mechanisms. Many studies have been published on neutrophil polarization, in which three different temperatures pretreatment (4 degrees C, 25 degrees C and 37 degrees C) have been used. However, no study has investigated whether different temperature pretreatments affect neutrophil polarization. In the current study, we examined the effects of 4 degrees C, 25 degrees C and 37 degrees C pretreatment temperatures on short-term (1 or 3 min) chemoattractant-induced polarization. Human neutrophils were polarized upon the stimulation of N-Formyl-Met-Leu-Phe (fMLP) after pretreated by different temperature. The morphological changes of the neutrophils were investigated under the microscopy. The F-actin polymerization was determined by immunological histological chemistry. There were more head-tail polarized cells (>50% of the cells) in the 25 degrees C and 37 degrees C pretreatment groups than in the 4 degrees C group (32.4%). The average lengths of the pseudopod were 3.2 +/- 1.1 microm (n = 17), 5.3 +/- 2.1 microm (n = 12) and 7.4 +/- 2.7 microm (n = 21) in the 4 degrees C, 25 degrees C and 37 degrees C pretreatment groups, respectively; the 4 degrees C and 37 degrees C pretreatment groups were statistically different (P < 0.05). Additionally, there was a statistically significant difference in the pseudopod extension rate among the three groups, as well as the Lamellipod percentage between the 4 degrees C group and the other two groups within 1 min of stimulation with fMLP. This study demonstrates that different temperature pretreatments affect neutrophil polarization upon short-term stimulation with fMLP.
N-formyl-Met-Leu-Phe-induced oxidative burst in DMSO-differentiated HL-60 cells requires active Hsp90, but not intact microtubules.[Pubmed:15744362]
Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2004 Dec;148(2):141-4.
In this study we examined whether microtubules and heat shock protein 90 (Hsp90) are involved in phorbol myristate acetate (PMA) and N-Formyl-Met-Leu-Phe (fMLP)-induced oxidative burst in DMSO-differentiated HL-60 cells. Our results showed that microtubule interfering agents, paclitaxel (1-5 microM), colchicine (1-100 microM), nocodazole (1-20 microM), and vincristine (1-50 microM), did not affect either PMA or fMLP-induced oxidative burst. In contrast, radicicol, an inhibitor of Hsp90, inhibited fMLP-induced oxidative burst in time and concentration-dependent manner where IC50 value for 30 min pre-incubation was 16.5 +/- 3.5 microM radicicol. We conclude that both PMA and fMLP-induced oxidative burst in DMSO-differentiated HL-60 cells is microtubule-independent while the latter requires Hsp90 activity.
Characterization of the binding site on the formyl peptide receptor using three receptor mutants and analogs of Met-Leu-Phe and Met-Met-Trp-Leu-Leu.[Pubmed:10960471]
J Biol Chem. 2000 Dec 15;275(50):39012-7.
The formyl peptide receptor (FPR) is a chemotactic G protein-coupled receptor found on the surface of phagocytes. We have previously shown that the formyl peptide binding site maps to the membrane-spanning region (Miettinen, H. M., Mills, J. S., Gripentrog, J. M., Dratz, E. A., Granger, B. L., and Jesaitis, A. J. (1997) J. Immunol. 159, 4045-4054). Recent reports have indicated that non-formylated peptides, such as MMWLL can also activate this receptor (Chen, J., Bernstein, H. S., Chen, M., Wang, L., Ishi, M., Turck, C. W., and Coughlin, S. R. (1995) J. Biol. Chem. 270, 23398-23401.) Here we show that the selectivity for the binding of different NH(2)-terminal analogs of MMWLL or MLF can be markedly altered by mutating Asp-106 to asparagine or Arg-201 to alanine. Both D106N and R201A produced a similar change in ligand specificity, including an enhanced ability to bind the HIV-1 peptide DP178. In contrast, the mutation R205A exhibited altered specificity at the COOH terminus of fMLF, with R205A binding fMLF-O-butyl > fMLF-O-methyl > fMLF, whereas wt FPR bound fMLF > fMLF-O-methyl approximately fMLF-O-butyl. These data, taken together with our previous finding that the leucine side chain of fMLF is probably bound to FPR near FPR (93)VRK(95) (Mills, J. S., Miettinen, H. M., Barnidge, D., Vlases, M. J., Wimer-Mackin, S., Dratz, E. A., and Jesaitis, A. J. (1998) J. Biol. Chem. 273, 10428-10435.), indicate that the most likely positioning of fMLF in the binding pocket of FPR is approximately parallel to the fifth transmembrane helix with the formamide group of fMLF hydrogen-bonded to both Asp-106 and Arg-201, the leucine side chain pointing toward the second transmembrane region, and the COOH-terminal carboxyl group of fMLF ion-paired with Arg-205.
Receptor blockade as a mechanism of deactivation of human neutrophils by pepstatin and formyl-Met-Leu-Phe.[Pubmed:6325345]
Inflammation. 1984 Mar;8(1):73-86.
The pentapeptide pepstatin was shown to be chemotactic for human neutrophils by two techniques: ED50 for chemotaxis was found to be 3 microM by the agarose method and 0.2 microM by the Boyden chamber technique. Pepstatin also induced superoxide radical generation, release of lysosomal enzymes, and a transient increase in intercellular adenosine-3',5'-cyclic monophosphate (cAMP) levels in a dose-dependent manner. Carbobenzoxy-phenylalanyl-methionine (CBZ-PM), which competitively inhibits formyl-methionyl-leucyl-phenylalanine (FMLP) -induced neutrophil functions, also inhibited pepstatin-induced neutrophil function of superoxide generation in a dose-dependent fashion. Likewise, pepstatin inhibited the binding of [3H]FMLP to the cells. Furthermore, preincubation of neutrophils with suboptimal concentrations of FMLP or pepstatin diminished the cellular response toward either factor when tested for their chemotactic activity and for their ability to induce superoxide generation, to release granule enzymes, and to induce a transient increase in intracellular cAMP levels. The concentrations of pepstatin or FMLP tested had no effect on superoxide generation, granule enzyme release, or intracellular levels of cAMP on subsequent challenge with C5a; both of these factors, however, cross-deactivated the chemotactic response of the cells towards C5a. Similar results were observed when cells were preincubated with C5a and subsequently challenged with pepstatin or FMLP. These results suggest that FMLP and pepstatin interact with the same receptor molecules to activate human neutrophil functions. Furthermore, our data indicate that the deactivation of the neutrophil functions of superoxide production and granule enzyme release are receptor specific, but the heterologous deactivation of chemotaxis involves a postreceptor mechanism(s).
Quantitative comparisons of various biological responses of neutrophils to different active and inactive chemotactic factors.[Pubmed:45788]
Immunopharmacology. 1978 Dec;1(1):39-47.
The effects of chemotactic factors on rabbit neutrophils were evaluated measuring cell migration in modified Boyden chambers and under agarose, in lysosomal enzyme release, leukocyte aggregation, and in vivo neutropenia. Chemotactins employed included the complement-derived C3 and C5 fragments, the bacterial chemotactic factor from culture supernatant fluids of Escherichia coli, and the synthetic chemotactic factors Met-Leu-Phe and formyl-Met-Leu-Phe. A consistent parallelism was found in all the leukocyte responses to a given chemotactic factor. In no instance, with any of the five chemotactic factor preparations, did cells responding in one assay system fail to respond in the four other assay systems, suggesting a common event in all of the cell responses. Boyden chamber chemotaxis was consistently the most sensitive assay; the agarose assay was, in general, less sensitive by a factor of 100 fold. Enzyme release approached, in cell sensitivity to chemotactic factors, that of the Boyden chamber assay. In general, in vitro leukocyte aggregation and in vivo neutropenia were considerably less sensitive assays. Chemotactic factor inactivator (CFI) purified from human serum destroyed in parallel all biological activities of C3 and C5 chemotactic factors but had no effect on the bacterial chemotactic factor and the activities of synthetic chemotactic peptides.


