GlabridinCAS# 59870-68-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 59870-68-7 | SDF | Download SDF |
| PubChem ID | 124052 | Appearance | White powder |
| Formula | C20H20O4 | M.Wt | 324.37 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | DMSO : 25 mg/mL (77.07 mM; Need ultrasonic) | ||
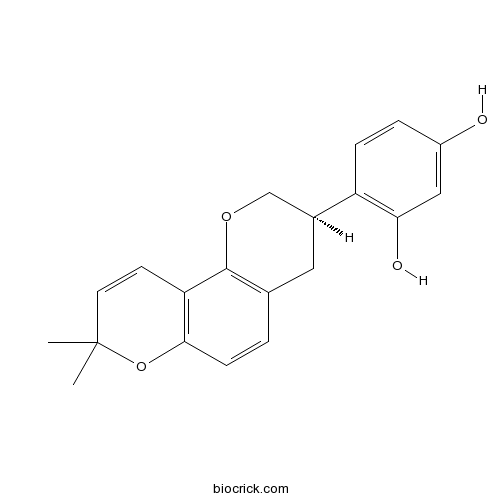
| Chemical Name | 4-[(3R)-8,8-dimethyl-3,4-dihydro-2H-pyrano[2,3-f]chromen-3-yl]benzene-1,3-diol | ||
| SMILES | CC1(C=CC2=C(O1)C=CC3=C2OCC(C3)C4=C(C=C(C=C4)O)O)C | ||
| Standard InChIKey | LBQIJVLKGVZRIW-ZDUSSCGKSA-N | ||
| Standard InChI | InChI=1S/C20H20O4/c1-20(2)8-7-16-18(24-20)6-3-12-9-13(11-23-19(12)16)15-5-4-14(21)10-17(15)22/h3-8,10,13,21-22H,9,11H2,1-2H3/t13-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Glabridin is a GABAA receptor positive modulator promoting fatty acid oxidation and improving learning and memory, which has antioxidative,anti-inflammatory, antimalarial, estrogen receptor agonism, anti-metastasis, anti-melanogenesis and neuroprotective effects. Glabridin may possess a therapeutic effect on metabolic disorders( such as diabetes and hyperglycemia), by modulating glucose metabolism through AMPK in skeletal muscle cells. |
| Targets | NOS | MMP(e.g.TIMP) | ERK | JNK | NF-kB | AMPK | Akt | GLUT | LDL | GABAA receptor |
| In vitro | Glabridin inhibits migration and invasion by transcriptional inhibition of matrix metalloproteinase 9 through modulation of NF-κB and AP-1 activity in human liver cancer cells.[Pubmed: 24641665]Br J Pharmacol. 2014 Jun;171(12):3037-50.High mortality and morbidity rates for hepatocellular carcinoma in Taiwan primarily result from uncontrolled tumour metastasis. Glabridin, a prenylated isoflavonoid of licorice (Glycyrrhiza glabra) roots, is associated with a wide range of biological properties, such as regulation of energy metabolism, oestrogenic, neuroprotective, anti-osteoporotic and skin whitening. However, the effect of Glabridin on the metastasis of tumour cells has not been clarified.
Glabridin induces oxidative stress mediated apoptosis like cell death of malaria parasite Plasmodium falciparum.[Pubmed: 24361284]Parasitol Int. 2014 Apr;63(2):349-58.Plants are known as the source of novel agents for developing new antimalarial drugs. Glabridin is a polyphenolic flavonoid, a main constituent in the roots of Glycyrrhiza glabra possesses various biological activities. However, its anti-plasmodial activity is unexplored.
|
| In vivo | Glabridin, an isoflavan from licorice root, downregulates iNOS expression and activity under high-glucose stress and inflammation.[Pubmed: 25737160]Mol Nutr Food Res. 2015 Mar 3.In females, hyperglycemia abolishes estrogen-vascular protection, leading to inflammation and oxidative stress that are related to diabetes-associated cardiovascular complications. Such knowledge led us to examine the potential of Glabridin, as a replacement of estrogen anti-inflammatory activity under high-glucose conditions.
Estrogenic and antiproliferative properties of glabridin from licorice in human breast cancer cells.[Pubmed: 11059763]Cancer Res. 2000 Oct 15;60(20):5704-9.
|
| Cell Research | Glabridin induces glucose uptake via the AMP-activated protein kinase pathway in muscle cells.[Pubmed: 24953974]Mol Cell Endocrinol. 2014 Aug 5;393(1-2):99-108.
|
| Animal Research | In vitro and in vivo neuroprotective effect and mechanisms of glabridin, a major active isoflavan from Glycyrrhiza glabra (licorice).[Pubmed: 18048062]Life Sci. 2008 Jan 2;82(1-2):68-78.Stroke is a life-threatening disease characterized by rapidly developing clinical signs of focal or global disturbance of cerebral function due to cerebral ischemia. A number of flavonoids have been shown to attenuate the cerebral injuries in stroked animal models. Glabridin, a major flavonoid of Glycyrrhiza glabra (licorice), possesses multiple pharmacological activities. This study aimed to investigate whether Glabridin modulated the cerebral injuries induced by middle cerebral artery occlusion (MCAO) in rats and staurosporine-induced damage in cultured rat cortical neurons and the possible mechanisms involved. |
| Structure Identification | Pigment Cell Res. 1998 Dec;11(6):355-61.The inhibitory effect of glabridin from licorice extracts on melanogenesis and inflammation.[Pubmed: 9870547]Glabridin is the main ingredient in hydrophobic fraction of licorice extract affecting on skins.
|

Glabridin Dilution Calculator

Glabridin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0829 mL | 15.4145 mL | 30.829 mL | 61.658 mL | 77.0725 mL |
| 5 mM | 0.6166 mL | 3.0829 mL | 6.1658 mL | 12.3316 mL | 15.4145 mL |
| 10 mM | 0.3083 mL | 1.5414 mL | 3.0829 mL | 6.1658 mL | 7.7072 mL |
| 50 mM | 0.0617 mL | 0.3083 mL | 0.6166 mL | 1.2332 mL | 1.5414 mL |
| 100 mM | 0.0308 mL | 0.1541 mL | 0.3083 mL | 0.6166 mL | 0.7707 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Oxybuprocaine HCl
Catalog No.:BCC4691
CAS No.:5987-82-6
- Cyclosporin A
Catalog No.:BCC4773
CAS No.:59865-13-3
- Patchouli alcohol
Catalog No.:BCN4966
CAS No.:5986-55-0
- Palustrol
Catalog No.:BCN4100
CAS No.:5986-49-2
- Synephrine HCl
Catalog No.:BCC4359
CAS No.:5985-28-4
- Ajmalimine
Catalog No.:BCN3420
CAS No.:59846-31-0
- Tenoxicam
Catalog No.:BCC4733
CAS No.:59804-37-4
- UK 14,304
Catalog No.:BCC5226
CAS No.:59803-98-4
- Vindolinine
Catalog No.:BCN7822
CAS No.:5980-02-9
- Cyclosporin C
Catalog No.:BCC8160
CAS No.:59787-61-0
- 6beta-Angeloyloxy-1beta,10beta-epoxy-9-oxofuranoeremophilane
Catalog No.:BCN7600
CAS No.:59780-08-4
- Boc-Asp(OMe)-OH.DCHA
Catalog No.:BCC3367
CAS No.:59768-74-0
- L-Dihydroorotic acid
Catalog No.:BCC9007
CAS No.:5988-19-2
- N-Formyl-Met-Leu-Phe
Catalog No.:BCC7205
CAS No.:59880-97-6
- ent-7alpha,9-Dihydroxy-15-oxokaur-16-en-19,6bet-olide
Catalog No.:BCN7401
CAS No.:59885-89-1
- 7-hydroxy-4-benzopyrone
Catalog No.:BCC9209
CAS No.:59887-89-7
- Loliolid
Catalog No.:BCN3655
CAS No.:5989-02-6
- Mesopsin
Catalog No.:BCN8050
CAS No.:5989-16-2
- Medicagenic acid
Catalog No.:BCN3893
CAS No.:599-07-5
- 3,3'-Sulfonyldianiline
Catalog No.:BCC8595
CAS No.:599-61-1
- Sulfasalazine
Catalog No.:BCC2545
CAS No.:599-79-1
- 3-Hydroxy-8,9-methylenedioxypterocarpene
Catalog No.:BCN1407
CAS No.:59901-98-3
- Vicenin -3
Catalog No.:BCN3014
CAS No.:59914-91-9
- HPI 1
Catalog No.:BCC3938
CAS No.:599150-20-6
Glabridin induces glucose uptake via the AMP-activated protein kinase pathway in muscle cells.[Pubmed:24953974]
Mol Cell Endocrinol. 2014 Aug 5;393(1-2):99-108.
The present study demonstrates that Glabridin, a prenylated isoflavone in licorice, stimulates glucose uptake through the adenosine monophosphate-activated protein kinase (AMPK) pathway in L6 myotubes. Treatment with Glabridin for 4h induced glucose uptake in a dose-dependent manner accompanied by the translocation of glucose transporter type 4 (GLUT4) to the plasma membrane. Glabridin needed at least 4h to increase glucose uptake, while it significantly decreased glycogen and increased lactic acid within 15 min. Pharmacological inhibition of AMPK by Compound C suppressed the Glabridin-induced glucose uptake, whereas phosphoinositide 3-kinase and Akt inhibition by LY294002 and Akt1/2 inhibitor, respectively, did not. Furthermore, Glabridin induced AMPK phosphorylation, and siRNA for AMPK completely abolished Glabridin-induced glucose uptake. We confirmed that Glabridin-rich licorice extract prevent glucose intolerance accompanied by the AMPK-dependent GLUT4 translocation in the plasma membrane of mice skeletal muscle. These results indicate that Glabridin may possess a therapeutic effect on metabolic disorders, such as diabetes and hyperglycemia, by modulating glucose metabolism through AMPK in skeletal muscle cells.
Glabridin induces oxidative stress mediated apoptosis like cell death of malaria parasite Plasmodium falciparum.[Pubmed:24361284]
Parasitol Int. 2014 Apr;63(2):349-58.
Plants are known as the source of novel agents for developing new antimalarial drugs. Glabridin is a polyphenolic flavonoid, a main constituent in the roots of Glycyrrhiza glabra possesses various biological activities. However, its anti-plasmodial activity is unexplored. In the present work, it is for the first time demonstrated that Glabridin inhibits Plasmodium falciparum growth in vitro with an IC50 23.9+/-0.43muM. Glabridin showed poor cytotoxicity in vitro with an IC50 246.6+/-0.88muM against Vero cell line and good selectivity index (9.6). In erythrocytic cycle, trophozoite stage was found to be most sensitive to Glabridin. In silico study showed that Glabridin inhibits Pf LDH enzyme activity by acting on NADH binding site. Glabridin induced oxidative stress by the generation of reactive oxygen and nitrogen species. Glabridin could induce apoptosis in parasite as evidenced by the depolarization of mitochondrial membrane potential (Deltapsim), activation of caspase like proteases and DNA fragmentation. These results indicate that Glabridin exhibits antiplasmodial activity and is suitable for developing antimalarial agent from a cheap and sustainable source.
In vitro and in vivo neuroprotective effect and mechanisms of glabridin, a major active isoflavan from Glycyrrhiza glabra (licorice).[Pubmed:18048062]
Life Sci. 2008 Jan 2;82(1-2):68-78.
Stroke is a life-threatening disease characterized by rapidly developing clinical signs of focal or global disturbance of cerebral function due to cerebral ischemia. A number of flavonoids have been shown to attenuate the cerebral injuries in stroked animal models. Glabridin, a major flavonoid of Glycyrrhiza glabra (licorice), possesses multiple pharmacological activities. This study aimed to investigate whether Glabridin modulated the cerebral injuries induced by middle cerebral artery occlusion (MCAO) in rats and staurosporine-induced damage in cultured rat cortical neurons and the possible mechanisms involved. Our study showed that Glabridin at 25mg/kg by intraperitoneal injection, but not at 5mg/kg, significantly decreased the focal infarct volume, cerebral histological damage and apoptosis in MCAO rats compared to sham-operated rats. Glabridin significantly attenuated the level of brain malonyldialdehyde (MDA) in MCAO rats, while it elevated the level of two endogenous antioxidants in the brain, i.e. superoxide dismutase (SOD) and reduced glutathione (GSH). Co-treatment with Glabridin significantly inhibited the staurosporine-induced cytotoxicity and apoptosis of cultured rat cortical neurons in a concentration-dependent manner. Consistently, Glabridin significantly reduced the DNA laddering caused by staurosporine in a concentration-dependent manner. Glabridin also suppressed the elevated Bax protein and caspase-3 proenzyme and decreased bcl-2 induced by staurosporine in cultured rat cortical neurons, facilitating cell survival. Glabridin also inhibited superoxide production in cultured cortical neurons exposed to staurosporine. These findings indicated that Glabridin had a neuroprotective effect via modulation of multiple pathways associated with apoptosis. Further studies are warranted to further investigate the biochemical mechanisms for the protective effect of Glabridin on neurons and the evidence for clinical use of licorice in the management of cerebral ischemia.
Glabridin, an isoflavan from licorice root, downregulates iNOS expression and activity under high-glucose stress and inflammation.[Pubmed:25737160]
Mol Nutr Food Res. 2015 Jun;59(6):1041-52.
SCOPE: In females, hyperglycemia abolishes estrogen-vascular protection, leading to inflammation and oxidative stress that are related to diabetes-associated cardiovascular complications. Such knowledge led us to examine the potential of Glabridin, as a replacement of estrogen anti-inflammatory activity under high-glucose conditions. METHODS AND RESULTS: In macrophage-like cells, chronic glucose stress (28 and 44 mM) upregulated inducible nitric oxide synthase (iNOS) mRNA expression by 42 and 189%, respectively. Pretreatment with Glabridin, under chronic glucose stress, downregulated the LPS-induced nitric oxide secretion and nitrotyrosine formation, by 39 and 21%, respectively. Pretreatment with estradiol did not prevent the LPS-induced nitrotyrosine formation. Furthermore, Glabridin, brought about a decrease in the LPS-induced iNOS mRNA expression by 48%, as compared to cells pretreated with estradiol. Glabridin decreased protein levels of liver iNOS by 69% in adult mouse offspring which developed hyperglycemia after early fetal exposure to a saturated fatty acid-enriched maternal diet. Glabridin also decreased liver nitrotyrosine levels in offspring of regular diet-fed mothers after further receiving high-fat diet. CONCLUSION: Such results indicate that Glabridin retains anti-inflammatory abilities to regulate the synthesis and activity of iNOS under high-glucose levels, implying that a Glabridin supplement may serve as an anti-inflammatory agent in diabetes-related vascular dysfunction.
Estrogenic and antiproliferative properties of glabridin from licorice in human breast cancer cells.[Pubmed:11059763]
Cancer Res. 2000 Oct 15;60(20):5704-9.
There is an increasing demand for natural compounds that improve women's health by mimicking the critical benefits of estrogen to the bones and the cardiovascular system but avoiding its deleterious effects on the breast and uterus. The estrogenic properties of Glabridin, the major isoflavan in licorice root, were tested in view of the resemblance of its structure and lipophilicity to those of estradiol. The results indicate that Glabridin is a phytoestrogen, binding to the human estrogen receptor and stimulating creatine kinase activity in rat uterus, epiphyseal cartilage, diaphyseal bone, aorta, and left ventricle of the heart. The stimulatory effects of 2.5-25 microg/animal Glabridin were similar to those of 5 microg/animal estradiol. Chemical modification of Glabridin showed that the position of the hydroxyl groups has a significant role in binding to the human estrogen receptor and in proliferation-inducing activity. Glabridin was found to be three to four times more active than 2'-O-methylGlabridin and 4'-O-methylGlabridin, and both derivatives were more active than 2',4'-O-methylGlabridin. The effect of increasing concentrations of Glabridin on the growth of breast tumor cells was biphasic. Glabridin showed an estrogen receptor-dependent, growth-promoting effect at low concentrations (10 nM-10 microM) and estrogen receptor-independent antiproliferative activity at concentrations of > 15 microM. This is the first study to indicate that isoflavans have estrogen-like activities. Glabridin and its derivatives exhibited varying degrees of estrogen receptor agonism in different tests and demonstrated growth-inhibitory actions on breast cancer cells.
The antioxidative effects of the isoflavan glabridin on endogenous constituents of LDL during its oxidation.[Pubmed:9568736]
Atherosclerosis. 1998 Mar;137(1):49-61.
The effect of the consumption of Glabridin, an isoflavan isolated from Glycyrrhiza glabra (licorice) root, on the susceptibility of low density lipoprotein (LDL) to oxidation was studied in atherosclerotic apolipoprotein E deficient (E[o] mice) and was compared with that of the known flavonoids, quercetin and catechin. Glabridin inhibitory activity on in vitro oxidation of human LDL was also investigated by determining the formation of lipid peroxides and oxysterols and the consumption of LDL-associated lipophilic antioxidants. Determination of the extent of LDL oxidation by measuring the formation of thiobabituric acid reactive substances (TBARS) after 2 h of LDL incubation with CuSO4 (10 microM) or 2,2'-azobis (2-amidino-propane) dihydrochloride (AAPH) (5 mM), revealed that Glabridin or quercetin consumption resulted in a 53 and 54% reduction in copper ion induced oxidation, respectively, and a 95 and 83% reduction in AAPH induced LDL oxidation, respectively. No inhibition was obtained with consumption of catechin. About 80% of Glabridin was found to bind to the LDL human particle. In the in vitro oxidation of LDL induced by AAPH (5 mM), Glabridin inhibited the formation of TBARS, lipid peroxides and cholesteryl linoleate hydroperoxide (CLOOH) at all the concentrations tested (5-60 microM), while in oxidation induced by copper ions (10 microM), Glabridin exhibited a pro-oxidant activity at concentrations lower than 20 microM, and a clear antioxidant activity at concentrations greater than 20 microM. Glabridin (30 microM) inhibited the formation of cholest-5-ene-3,7-diol (7-hydroxycholesterol), cholest-5-ene-3-ol-7-one (7-ketocholesterol) and cholestan-5,6-epoxy-3-ol (5,6-epoxycholesterol) after 6 h of AAPH induced LDL oxidation, by 55, 80 and 40%, respectively, and after 6 h of copper ion induced LDL oxidation, by 73, 94 and 52%, respectively. Glabridin also inhibited the consumption of beta-carotene and lycopene by 38 and 52%, respectively, after 0.5 h of LDL oxidation with AAPH, but failed to protect vitamin E. The in vivo and in vitro reduction of the susceptibility of LDL to oxidation obtained with Glabridin, may be related to the absorption or binding of Glabridin to the LDL particle and subsequent protection of LDL from oxidation by inhibiting the formation of lipid peroxides and oxysterols, and by protecting LDL associated carotenoids.
The inhibitory effect of glabridin from licorice extracts on melanogenesis and inflammation.[Pubmed:9870547]
Pigment Cell Res. 1998 Dec;11(6):355-61.
Glabridin is the main ingredient in hydrophobic fraction of licorice extract affecting on skins. In this study, we investigated inhibitory effects of Glabridin on melanogenesis and inflammation using cultured B16 murine melanoma cells and guinea pig skins. The results indicated that Glabridin inhibits tyrosinase activity of these cells at concentrations of 0.1 to 1.0 microg/ml and had no detectable effect on their DNA synthesis. Combined analysis of SDS-polyacrylamide gel electrophoresis and DOPA staining on the large granule fraction of these cells disclosed that Glabridin decreased specifically the activities of T1 and T3 tyrosinase isozymes. It was also shown that UVB-induced pigmentation and erythema in the skins of guinea pigs were inhibited by topical applications of 0.5% Glabridin. Anti-inflammatory effects of Glabridin in vitro were also shown by its inhibition of superoxide anion productions and cyclooxygenase activities. These data indicated that Glabridin is a unique compound possessing more than one function; not only the inhibition of melanogenesis but also the inhibition of inflammation in the skins. By replacing each of hydroxyl groups of Glabridin with others, it was revealed that the inhibitory effect of 2'-O-ethyl Glabridin was significantly stronger than that of 4'-O-ethyl-Glabridin on melanin synthesis in cultured B16 cells at the concentration of 1.0 mg/ml. With replacement of both of two hydroxyl groups, the inhibitory effect was totally lost. Based on these data, we concluded that two hydroxyl groups of Glabridin are important for the inhibition of melanin synthesis and that the hydroxyl group at the 4' position of this compound is more closely related to melanin synthesis.
Glabridin inhibits migration and invasion by transcriptional inhibition of matrix metalloproteinase 9 through modulation of NF-kappaB and AP-1 activity in human liver cancer cells.[Pubmed:24641665]
Br J Pharmacol. 2014 Jun;171(12):3037-50.
BACKGROUND AND PURPOSE: High mortality and morbidity rates for hepatocellular carcinoma in Taiwan primarily result from uncontrolled tumour metastasis. Glabridin, a prenylated isoflavonoid of licorice (Glycyrrhiza glabra) roots, is associated with a wide range of biological properties, such as regulation of energy metabolism, oestrogenic, neuroprotective, anti-osteoporotic and skin whitening. However, the effect of Glabridin on the metastasis of tumour cells has not been clarified. EXPERIMENTAL APPROACH: A wound healing model and Boyden chamber assays in vitro were used to determine the effects of Glabridin on the migration and invasion of human hepatocellular carcinoma (HHC) cells. Western blot analysis, gelatin zymography, real-time PCR and promoter assays were used to evaluate the inhibitory effects of Glabridin on matrix metalloproteinase 9 (MMP9) expression in these cells. KEY RESULTS: Glabridin significantly inhibited migration/invasion capacities of HCC cells, Huh7 and Sk-Hep-1, cell lines that have low cytotoxicity in vitro, even at high concentrations. Western blot analysis and gelatin zymography showed that Glabridin inhibited the expression, activities and protein levels of MMP9 and the phosphorylation of ERK1/2 and JNK1/2. These inhibitory effects were associated with an up-regulation of tissue inhibitor of metalloproteinase-1 and a down-regulation of the transcription factors NF-kappaB and activator protein 1 signalling pathways. Finally, the administration of Glabridin effectively suppressed the tumour formation in the hepatoma xenograft model in vivo. CONCLUSION AND IMPLICATIONS: Glabridin inhibited the invasion of human HCC cells and may have potential as a chemopreventive agent against liver cancer metastasis.


