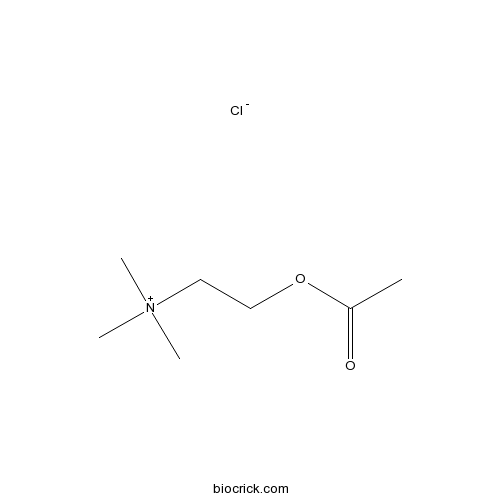
Acetylcholine chlorideMajor transmitter at many nervous sites CAS# 60-31-1 |
- Etifoxine
Catalog No.:BCC1560
CAS No.:21715-46-8
- Etomidate
Catalog No.:BCC1150
CAS No.:33125-97-2
- Etifoxine hydrochloride
Catalog No.:BCC1561
CAS No.:56776-32-0
- Flumazenil
Catalog No.:BCC1259
CAS No.:78755-81-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 60-31-1 | SDF | Download SDF |
| PubChem ID | 6060 | Appearance | White powder |
| Formula | C7H16ClNO2 | M.Wt | 181.66 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Ach; ACh chloride | ||
| Solubility | H2O : 500 mg/mL (2752.39 mM; Need ultrasonic) DMSO : ≥ 30 mg/mL (165.14 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 2-acetyloxyethyl(trimethyl)azanium;chloride | ||
| SMILES | CC(=O)OCC[N+](C)(C)C.[Cl-] | ||
| Standard InChIKey | JUGOREOARAHOCO-UHFFFAOYSA-M | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Acetylcholine Chloride is a neurotransmitter in both the peripheral nervous system (PNS) and central nervous system (CNS) in many organisms including humans. Acetylcholine chloride in micromolar concentrations significantly inhibit p53 mutant peptide aggregation in vitro, and could be promising candidate for p53 mutant/ misfolded protein aggregation inhibition, and mutations of tumor suppressor protein p53 are present in almost about 50% of all cancers. |
| Targets | p53 | AChR |
| In vitro | Inhibition of p53 Mutant Peptide Aggregation In Vitro by Cationic Osmolyte Acetylcholine Chloride.[Pubmed: 28117010]Protein Pept Lett. 2017;24(4):353-357.Mutations of tumor suppressor protein p53 are present in almost about 50% of all cancers. It has been reported that the p53 mutations cause aggregation and subsequent loss of p53 function, leading to cancer progression. |
| In vivo | Acetylcholine chloride as a potential source of variability in the study of cutaneous vascular function in man.[Pubmed: 21601579]Microvasc Res. 2011 Sep;82(2):190-7. Laser-Doppler flowmetry (LDF) coupled with Acetylcholine chloride (ACh) iontophoresis is increasingly recognized as a reliable non-invasive method to study the endothelial function. However, Acetylcholine chloride-vasodilation measurement appears highly variable possibly due to the Acetylcholine chloride pharmacological properties itself. These problems may be partially overcome by using methacholine chloride (MCh), a more stable synthetic agonist of muscarinic receptors, instead of Acetylcholine chloride. Therefore, we first studied the correlation between the two drugs and then the effects of (1) spatial variability (inter-site measurements), (2) temporal variability (inter-day measurements), (3) intra-day variability (morning versus evening), and (4) age on the variability of both Acetylcholine chloride-vasodilation and MCh-vasodilation. |

Acetylcholine chloride Dilution Calculator

Acetylcholine chloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.5048 mL | 27.5239 mL | 55.0479 mL | 110.0958 mL | 137.6197 mL |
| 5 mM | 1.101 mL | 5.5048 mL | 11.0096 mL | 22.0192 mL | 27.5239 mL |
| 10 mM | 0.5505 mL | 2.7524 mL | 5.5048 mL | 11.0096 mL | 13.762 mL |
| 50 mM | 0.1101 mL | 0.5505 mL | 1.101 mL | 2.2019 mL | 2.7524 mL |
| 100 mM | 0.055 mL | 0.2752 mL | 0.5505 mL | 1.101 mL | 1.3762 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Acetylcholine in vertebrates is the major transmitter at neuromuscular junctions, autonomic ganglia, parasympathetic effector junctions, a subset of sympathetic effector junctions, and at many sites in the central nervous system. It is generally not used
- H-Tyr-OH
Catalog No.:BCC3123
CAS No.:60-18-4
- Guanethidine Sulfate
Catalog No.:BCC3789
CAS No.:60-02-6
- EDTA
Catalog No.:BCC7493
CAS No.:60-00-4
- Tagitinin A
Catalog No.:BCN4102
CAS No.:59979-61-2
- Tagitinin F
Catalog No.:BCN4101
CAS No.:59979-57-6
- Carlinoside
Catalog No.:BCN2853
CAS No.:59952-97-5
- Malotilate
Catalog No.:BCC1196
CAS No.:59937-28-9
- 3',4'-dihydro-3'-hydroxy-Xanthyletin
Catalog No.:BCN3680
CAS No.:5993-18-0
- Vicriviroc maleate
Catalog No.:BCC2038
CAS No.:599179-03-0
- Vindesine sulfate
Catalog No.:BCC8266
CAS No.:59917-39-4
- HPI 1
Catalog No.:BCC3938
CAS No.:599150-20-6
- Vicenin -3
Catalog No.:BCN3014
CAS No.:59914-91-9
- (6-)ε-Aminocaproic acid
Catalog No.:BCC4888
CAS No.:60-32-2
- Linoleic acid
Catalog No.:BCN3821
CAS No.:60-33-3
- Acetamide
Catalog No.:BCN4114
CAS No.:60-35-5
- Acetyl-Strophanthidin
Catalog No.:BCC8113
CAS No.:60-38-8
- Tetracycline
Catalog No.:BCC9176
CAS No.:60-54-8
- Methimazole
Catalog No.:BCC3812
CAS No.:60-56-0
- Veratramine
Catalog No.:BCN2965
CAS No.:60-70-8
- Antipyrine
Catalog No.:BCC8834
CAS No.:60-80-0
- Phlorizin
Catalog No.:BCN4126
CAS No.:60-81-1
- Phloretin
Catalog No.:BCN4128
CAS No.:60-82-2
- Adenosine cyclophosphate
Catalog No.:BCN2190
CAS No.:60-92-4
- N-Me-DL-Ala-OH.HCl
Catalog No.:BCC2618
CAS No.:600-21-5
Acetylcholine chloride as a potential source of variability in the study of cutaneous vascular function in man.[Pubmed:21601579]
Microvasc Res. 2011 Sep;82(2):190-7.
INTRODUCTION: Laser-Doppler flowmetry (LDF) coupled with Acetylcholine chloride (ACh) iontophoresis is increasingly recognized as a reliable non-invasive method to study the endothelial function. However, ACh-vasodilation measurement appears highly variable possibly due to the ACh pharmacological properties itself. These problems may be partially overcome by using methacholine chloride (MCh), a more stable synthetic agonist of muscarinic receptors, instead of ACh. Therefore, we first studied the correlation between the two drugs and then the effects of (1) spatial variability (inter-site measurements), (2) temporal variability (inter-day measurements), (3) intra-day variability (morning versus evening), and (4) age on the variability of both ACh-vasodilation and MCh-vasodilation. METHODS: The endothelium-dependent vasodilation response to simultaneous iontophoretic applications (4 doses of 10s at 0.1mA with 2min of current-free interval) of ACh (11mM) or MCh (10mM) was studied on the forearm of 40 healthy subjects (36 males, median 28yr, range 21-59yr). The percent change in perfusion (CVCpeak) from baseline and the area under the curve (CVC(AUC)) during iontophoresis were assessed. Inter-site, inter-day and intra-day coefficients of variation (CV) were studied for each drug as well as correlations between drugs and age. RESULTS: A linear relationship was found between ACh- and MCh-CVCpeak (r(2)=0.75, p=0.01) and between ACh- and MCh-CVC(AUC) (r(2)=0.55, p=0.02). MCh inter-site CV for both CVCpeak (12.2%) and CVC(AUC) (13.8%) was significantly lower than ACh inter-site CV for CVCpeak (15.5%) and CVC(AUC) (15.3%), respectively. MCh inter-day CV for CVCpeak (17.2%) and CVC(AUC) (14.6%) was significantly lower than ACh inter-day CV for CVCpeak (19.7%) and ACh CVC(AUC) (21.2%). For ACh and MCh, the CVCpeak and CVC(AUC) were higher at 16:00pm than at 11:00am (p<0.05 for all). Finally, both ACh- and MCh-CVCpeak exhibited a logarithmic decrease with age (r(2)=0.61, p<0.01 and r(2)=0.58, p<0.01). CONCLUSION: Although both drugs exhibited circadian and age variability, MCh exhibited less inter-site and interday variabilities than did ACh for the evaluation of cutaneous endothelium-dependent vasodilation. These findings should be taken into account in studies of cutaneous vascular function by iontophoresis coupled with laser Doppler flowmetry.
Inhibition of p53 Mutant Peptide Aggregation In Vitro by Cationic Osmolyte Acetylcholine Chloride.[Pubmed:28117010]
Protein Pept Lett. 2017;24(4):353-357.
Mutations of tumor suppressor protein p53 are present in almost about 50% of all cancers. It has been reported that the p53 mutations cause aggregation and subsequent loss of p53 function, leading to cancer progression. Here in this study we focus on the inhibitory effects of cationic osmolyte molecules Acetylcholine chloride, and choline on an aggregation prone 10 amino acid p53 mutant peptide WRPILTIITL, and the corresponding wildtype peptide RRPILTIITL in vitro. The characterization tools used for this study include Thioflavin- T (ThT) induced fluorescence, transmission electron microscopy (TEM), congo red binding, turbidity, dynamic light scattering (DLS), and cell viability assays. The results show that Acetylcholine chloride in micromolar concentrations significantly inhibit p53 mutant peptide aggregation in vitro, and could be promising candidate for p53 mutant/ misfolded protein aggregation inhibition.
The cholinergic 'pitfall': acetylcholine, a universal cell molecule in biological systems, including humans.[Pubmed:10081614]
Clin Exp Pharmacol Physiol. 1999 Mar;26(3):198-205.
1. Acetylcholine (ACh) represents one of the most exemplary neurotransmitters. In addition to its presence in neuronal tissue, there is increasing experimental evidence that ACh is widely expressed in pro- and eukaryotic non-neuronal cells. Thus, ACh has been detected in bacteria, algae, protozoa, tubellariae and primitive plants, suggesting an extremely early appearance of ACh in the evolutionary process. 2. In humans, ACh and/or the synthesizing enzyme, choline acetyltransferase, has been demonstrated in epithelial cells (airways, alimentary tract, urogenital tract, epidermis), mesothelial (pleura, pericardium) and endothelial and muscle cells. In addition, immune cells express the non-neuronal cholinergic system (i.e. the synthesis of ACh can be detected in human leucocytes (granulocytes, lymphocytes and macrophages)), as well as in rat microglia in vitro. 3. The widespread expression of non-neuronal ACh is accompanied by the ubiquitous expression of cholinesterase activity, which prevents ACh from acting as a classical hormone. 4. Non-neuronal ACh mediates its cellular actions in an auto- and paracrine manner via the activation of the widely expressed nicotinic and muscarinic acetylcholine receptors, which can interfere with virtually all cellular signalling pathways (ion channels and key enzymes). 5. Non-neuronal ACh appears to be involved in the regulation of basic cell functions, such as mitosis, cell differentiation, organization of the cytoskeleton, cell-cell contact, secretion and absorption. Non-neuronal ACh also plays a role in the regulation of immune functions. All these qualities together may mediate the so-called 'trophic property' of ACh. 6. Future experiments should be designed to analyse the cellular effects of ACh in greater detail. The involvement of the non-neuronal cholinergic system in the pathogenesis of chronic inflammatory diseases should be investigated to open up new therapeutic strategies.


