MalotilateStimulates hepatocyte regeneration CAS# 59937-28-9 |
- Zileuton
Catalog No.:BCC2515
CAS No.:111406-87-2
- Zileuton sodium
Catalog No.:BCC4216
CAS No.:118569-21-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 59937-28-9 | SDF | Download SDF |
| PubChem ID | 4006 | Appearance | Powder |
| Formula | C12H16O4S2 | M.Wt | 288.38 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | NKK 105 | ||
| Solubility | DMSO : ≥ 100 mg/mL (346.76 mM) *"≥" means soluble, but saturation unknown. | ||
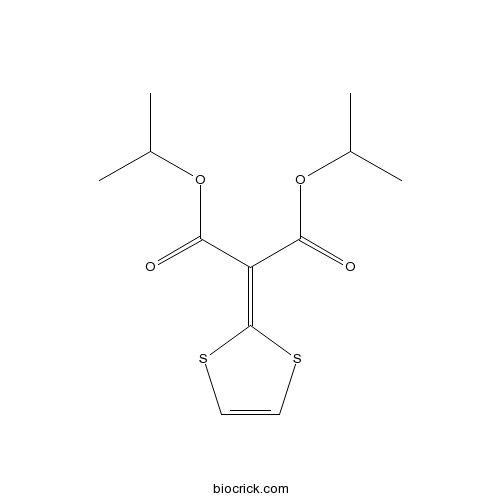
| Chemical Name | dipropan-2-yl 2-(1,3-dithiol-2-ylidene)propanedioate | ||
| SMILES | CC(C)OC(=O)C(=C1SC=CS1)C(=O)OC(C)C | ||
| Standard InChIKey | YPIQVCUJEKAZCP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H16O4S2/c1-7(2)15-10(13)9(11(14)16-8(3)4)12-17-5-6-18-12/h5-8H,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Malotilate is a liver protein metabolism improved compound, which selectively inhibit the 5-lipoxygenase.
IC50 Value:
Target: 5-lipoxygenase
in vitro: In an in vitro invasion assay using rat lung endothelial (RLE) cells, invasion of tumor cells which had been treated with MT (10 ng/ml, 24 h) was not affected; however, when RLE cells had been treated with MT, invasion was significantly inhibited in three cell lines (SAS, Ca9-22 and HSC-4) and a tendency to inhibition was also observed in other cell lines [1].
in vivo: The improvement rates for choline esterase activity were significantly greater in the malotilate group than in the control group. Serum albumin levels significantly increased in the malotilate group but not in the control group [2]. In the rats treated with MT for 19 days after i.v. inoculation of c-SST-2 cells, lung metastasis was also significantly suppressed [3]. Malotilate prevented increases in serum markers of type III and IV collagen synthesis as well as accumulation of the collagens, laminin and fibronectin in the liver [4].
Toxicity: Malotilate cytotoxicity to PBMCs, assessed by trypan blue dye exclusion and lactate dehydrogenase (LDH) release into the culture media, was found to be markedly increased by the addition of the NADPH generating system, indicating that metabolites play a significant role in toxicity [5]. References: | |||||

Malotilate Dilution Calculator

Malotilate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4676 mL | 17.3382 mL | 34.6765 mL | 69.3529 mL | 86.6912 mL |
| 5 mM | 0.6935 mL | 3.4676 mL | 6.9353 mL | 13.8706 mL | 17.3382 mL |
| 10 mM | 0.3468 mL | 1.7338 mL | 3.4676 mL | 6.9353 mL | 8.6691 mL |
| 50 mM | 0.0694 mL | 0.3468 mL | 0.6935 mL | 1.3871 mL | 1.7338 mL |
| 100 mM | 0.0347 mL | 0.1734 mL | 0.3468 mL | 0.6935 mL | 0.8669 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: N/A
Malotilate (diisopropyl-l,3-dithiol-2-ylidenemalonate) is a novel hepatotrophic drug, which can improve protein metabolism and stimulate hepatocyte regeneration.
In vitro: The in-vitro invasion assay employing a rat lung endothelial cell monolayer indicated that pretreatment of the lung endothelial cells, but not c-SST-2 cells, with malotilate reduced the invasion of the RLE monolayer by c-SST-2 cells significantly. The in-vitro vascular permeability assay also demonstrated malotilate could prevent the permeability increase of the lung endothelial cell monolayer by serum starvation. In contrast, the gelatinase production and adhesion to the RLE cell monolayer of c-SST-2 cells were not affected by malotilate treatment [1].
In vivo: Malotilate was administered to syngeneic SHR rats orally from 7 days before or after s.c. inoculation of c-SST-2 cells until the end of the experiments. In the malotilate-treated rats, pulmonary metastasis was suppressed markedly when compared with the non-treated rats. Moreover, in the rats treated with malotilate for 19 days after inoculation of c-SST-2 cells, lung metastasis was also suppressed significantly [1].
Clinical trial: A previous clinical trial results indicated that malotilate could accelerate the recovery of impaired protein metabolism in alcoholic liver disease and thus malotilate might be useful for the treatment of alcoholic liver diseases [2].
References:
[1] Nagayasu H,Hamada J,Kawano T,Konaka S,Nakata D,Shibata T,Arisue M,Hosokawa M,Takeichi N,Moriuchi T. Inhibitory effects of malotilate on invasion and metastasis of rat mammary carcinoma cells by modifying the functions of vascular endothelial cells. Br J Cancer.1998 May;77(9):1371-7.
[2] Takase S,Matsuda Y,Yasuhara M,Takada A. Effects of malotilate treatment on alcoholic liver disease. Alcohol.1989 May-Jun;6(3):219-22.
- 3',4'-dihydro-3'-hydroxy-Xanthyletin
Catalog No.:BCN3680
CAS No.:5993-18-0
- Vicriviroc maleate
Catalog No.:BCC2038
CAS No.:599179-03-0
- Vindesine sulfate
Catalog No.:BCC8266
CAS No.:59917-39-4
- HPI 1
Catalog No.:BCC3938
CAS No.:599150-20-6
- Vicenin -3
Catalog No.:BCN3014
CAS No.:59914-91-9
- 3-Hydroxy-8,9-methylenedioxypterocarpene
Catalog No.:BCN1407
CAS No.:59901-98-3
- Sulfasalazine
Catalog No.:BCC2545
CAS No.:599-79-1
- 3,3'-Sulfonyldianiline
Catalog No.:BCC8595
CAS No.:599-61-1
- Medicagenic acid
Catalog No.:BCN3893
CAS No.:599-07-5
- Mesopsin
Catalog No.:BCN8050
CAS No.:5989-16-2
- Loliolid
Catalog No.:BCN3655
CAS No.:5989-02-6
- 7-hydroxy-4-benzopyrone
Catalog No.:BCC9209
CAS No.:59887-89-7
- Carlinoside
Catalog No.:BCN2853
CAS No.:59952-97-5
- Tagitinin F
Catalog No.:BCN4101
CAS No.:59979-57-6
- Tagitinin A
Catalog No.:BCN4102
CAS No.:59979-61-2
- EDTA
Catalog No.:BCC7493
CAS No.:60-00-4
- Guanethidine Sulfate
Catalog No.:BCC3789
CAS No.:60-02-6
- H-Tyr-OH
Catalog No.:BCC3123
CAS No.:60-18-4
- Acetylcholine chloride
Catalog No.:BCN2197
CAS No.:60-31-1
- (6-)ε-Aminocaproic acid
Catalog No.:BCC4888
CAS No.:60-32-2
- Linoleic acid
Catalog No.:BCN3821
CAS No.:60-33-3
- Acetamide
Catalog No.:BCN4114
CAS No.:60-35-5
- Acetyl-Strophanthidin
Catalog No.:BCC8113
CAS No.:60-38-8
- Tetracycline
Catalog No.:BCC9176
CAS No.:60-54-8
Fibroblast-migration in a wound model of ascorbic acid-supplemented three-dimensional culture system: the effects of cytokines and malotilate, a new wound healing stimulant, on cell-migration.[Pubmed:9673894]
J Dermatol Sci. 1998 Jun;17(2):123-31.
To assess the migratory response of fibroblasts in vitro, normal human dermal fibroblasts (NHDF) were cultured in the presence of L-ascorbic acid 2-phosphate to induce a multilayered structure. Round wounds were made by punching, and the migratory response was evaluated by counting the number of migrating cells in the wounded areas. Collagenase activity in the culture-medium was then measured. When the wound model was treated with bFGF, IL-1 alpha or PDGF, the migratory response was facilitated with increased collagenase secretion. In contrast, treatment with TGF-beta reduced the migratory response and collagenase secretion. Since the multilayered structure is rich in collagenous matrix, degradation of the matrix by secreted collagenase is probably necessary for the cells to migrate into the wounded areas. Furthermore, Malotilate, which is now under development as an agent for wound therapy, facilitated the migratory response of NHDF with increased collagenase secretion in this wound model, suggesting that the wound healing effect of Malotilate is in part attributable to stimulated migration of fibroblasts to wounded areas subsequent to extracellular matrix-degradation.
Effect of malotilate on ethanol-induced gastric mucosal damage in capsaicin-pretreated rats.[Pubmed:10625227]
Physiol Res. 1999;48(5):375-81.
We studied the role of afferent sensory neurons in Malotilate-mediated gastric mucosal protection. Intact and capsaicin sensory-denervated rats were used in the experiments. Gross gastric mucosal injury was assessed and evaluated as a main criterion of the gastroprotective effect of the tested substances. Besides Malotilate, methyl-prostaglandin E2 was applied alone or in combination with Malotilate to compare the effects and the mechanism of action of both substances. The results revealed that both Malotilate as well as methyl-prostaglandin E2 exerted a significant protective action on 96% ethanol-induced gastric mucosal damage. However, there were no significant differences between intact and capsaicin-denervated rats. Only the use of 50% ethanol as a milder mucosal irritating agent resulted in significant differences in both groups of animals. We propose that Malotilate (like methyl-prostaglandin E2) has a gastroprotective effect on ethanol-induced gastric mucosal injury. This effect is partly dependent on the sensory nervous system and the combination of both above substances has an additive effect.
Inhibitory effects of malotilate on in vitro invasion of lung endothelial cell monolayer by human oral squamous cell carcinoma cells.[Pubmed:10940826]
Tumour Biol. 2000 Sep-Oct;21(5):299-308.
We have previously reported that Malotilate (MT) inhibited the invasion and metastasis of rat mammary carcinoma cells through the modification of host endothelial cells. In this study, we examined the inhibitory effects of MT on invasion of human cancer, using five oral squamous cell carcinoma cells (SAS, Ca9-22 and HSC-2, -3 and -4). MT did not affect the growth of these tumor cells and the invasion of reconstituted basement membrane, Matrigel. In an in vitro invasion assay using rat lung endothelial (RLE) cells, invasion of tumor cells which had been treated with MT (10 ng/ml, 24 h) was not affected; however, when RLE cells had been treated with MT, invasion was significantly inhibited in three cell lines (SAS, Ca9-22 and HSC-4) and a tendency to inhibition was also observed in other cell lines. Electron-microscopical examination of the RLE monolayer treated with MT (MT-RLE) showed the development of gap and tight junction-like structures. Subsequently, junction-associated proteins, connexin 43, zonula occludin and desmoglein, were examined by Western blotting. Protein levels of connexin 43 and zonula occludin were elevated dose dependently, and connexin 43 was chronologically enhanced by MT whereas desmoglein was not. The enhanced gap junctional communication of MT-RLE cells was observed in the scrape-loading assay using lucifer yellow CH. These results suggest that MT promotes the development of cell-to-cell adhesion, e. g. gap junction and tight junction in endothelial cells, resulting in the inhibition of invasion by the tumor cells.
Physicochemical and pharmacokinetic characterization of a spray-dried malotilate emulsion.[Pubmed:21619915]
Int J Pharm. 2011 Jul 29;414(1-2):186-92.
Malotilate (MT) is a hepatoprotective drug administered orally. However, MT was found to be a poorly water-soluble drug with low oral bioavailability. In the present investigation, a novel spray-dried emulsion (SDE) loaded with MT was prepared, and its physicochemical properties were characterized by rheological evaluation, particle size measurement, in vitro release, and surface morphology. The pharmacokinetic study of SDE, in comparison to MT suspension with the pure MT powder homogeneously dispersed in 0.5% CMC-Na solution, was also performed in rats after a single oral dose. It was found that SDE exhibited a 2.9-fold higher peak plasma concentration (C(max)) and 2.3-fold higher area under the curve (AUC) than MT suspension.


