TetracyclineCAS# 60-54-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 60-54-8 | SDF | Download SDF |
| PubChem ID | 54675776 | Appearance | Powder |
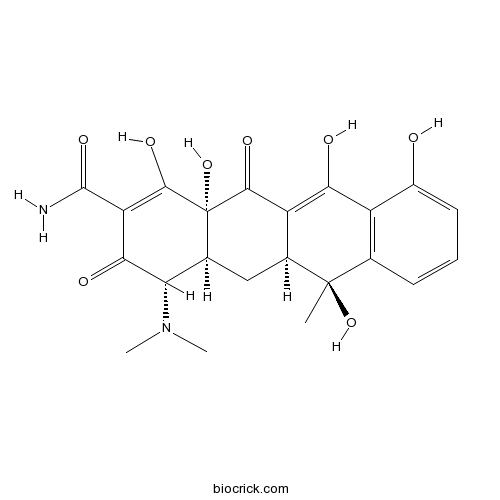
| Formula | C22H24N2O8 | M.Wt | 444.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 125 mg/mL (281.26 mM; Need ultrasonic) | ||
| Chemical Name | (4S,4aS,5aS,6S,12aR)-4-(dimethylamino)-1,6,10,11,12a-pentahydroxy-6-methyl-3,12-dioxo-4,4a,5,5a-tetrahydrotetracene-2-carboxamide | ||
| SMILES | CC1(C2CC3C(C(=O)C(=C(C3(C(=O)C2=C(C4=C1C=CC=C4O)O)O)O)C(=O)N)N(C)C)O | ||
| Standard InChIKey | NWXMGUDVXFXRIG-WESIUVDSSA-N | ||
| Standard InChI | InChI=1S/C22H24N2O8/c1-21(31)8-5-4-6-11(25)12(8)16(26)13-9(21)7-10-15(24(2)3)17(27)14(20(23)30)19(29)22(10,32)18(13)28/h4-6,9-10,15,25-26,29,31-32H,7H2,1-3H3,(H2,23,30)/t9-,10-,15-,21+,22-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Tetracycline Dilution Calculator

Tetracycline Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2502 mL | 11.2511 mL | 22.5023 mL | 45.0045 mL | 56.2556 mL |
| 5 mM | 0.45 mL | 2.2502 mL | 4.5005 mL | 9.0009 mL | 11.2511 mL |
| 10 mM | 0.225 mL | 1.1251 mL | 2.2502 mL | 4.5005 mL | 5.6256 mL |
| 50 mM | 0.045 mL | 0.225 mL | 0.45 mL | 0.9001 mL | 1.1251 mL |
| 100 mM | 0.0225 mL | 0.1125 mL | 0.225 mL | 0.45 mL | 0.5626 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Acetyl-Strophanthidin
Catalog No.:BCC8113
CAS No.:60-38-8
- Acetamide
Catalog No.:BCN4114
CAS No.:60-35-5
- Linoleic acid
Catalog No.:BCN3821
CAS No.:60-33-3
- (6-)ε-Aminocaproic acid
Catalog No.:BCC4888
CAS No.:60-32-2
- Acetylcholine chloride
Catalog No.:BCN2197
CAS No.:60-31-1
- H-Tyr-OH
Catalog No.:BCC3123
CAS No.:60-18-4
- Guanethidine Sulfate
Catalog No.:BCC3789
CAS No.:60-02-6
- EDTA
Catalog No.:BCC7493
CAS No.:60-00-4
- Tagitinin A
Catalog No.:BCN4102
CAS No.:59979-61-2
- Tagitinin F
Catalog No.:BCN4101
CAS No.:59979-57-6
- Carlinoside
Catalog No.:BCN2853
CAS No.:59952-97-5
- Malotilate
Catalog No.:BCC1196
CAS No.:59937-28-9
- Methimazole
Catalog No.:BCC3812
CAS No.:60-56-0
- Veratramine
Catalog No.:BCN2965
CAS No.:60-70-8
- Antipyrine
Catalog No.:BCC8834
CAS No.:60-80-0
- Phlorizin
Catalog No.:BCN4126
CAS No.:60-81-1
- Phloretin
Catalog No.:BCN4128
CAS No.:60-82-2
- Adenosine cyclophosphate
Catalog No.:BCN2190
CAS No.:60-92-4
- N-Me-DL-Ala-OH.HCl
Catalog No.:BCC2618
CAS No.:600-21-5
- 11Beta-hydroxyprogesterone
Catalog No.:BCN2211
CAS No.:600-57-7
- Oblongine
Catalog No.:BCN4103
CAS No.:60008-01-7
- Glabrene
Catalog No.:BCN6692
CAS No.:60008-03-9
- Chlormethiazole hydrochloride
Catalog No.:BCC6830
CAS No.:6001-74-7
- BOC-L-6-HYDROXYNORLEUCINE
Catalog No.:BCN2360
CAS No.:77611-37-1
Characterization of Erythromycin and Tetracycline Resistance Genes of Streptococcus gallolyticus Subspecies pasteurianus Strains Isolated from Patients with Septicemia and Bacteremia in Thailand.[Pubmed:30969077]
Clin Lab. 2019 Apr 1;65(4).
BACKGROUND: Streptococcus gallolyticus subspecies (subsp.) pasteurianus, previously known as Streptococcus bovis biotype II/2, has been described as a causative agent of endocarditis, neonatal sepsis, meningitis, bacteremia, and colorectal carcinoma in humans. The aim of this study was to characterize the erythromycin and Tetracycline resistance genes of S. gallolyticus subsp. pasteurianus strains isolated from patients with septicemia and bacteremia in Thailand. METHODS: The clinical isolates of Streptococcus gallolyticus were identified by using conventional biochemical tests, PCR, and sodA gene sequence analysis. The erythromycin and Tetracycline susceptibilities were determined by disk diffusion and agar dilution methods, while the resistance genes were identified by nucleotide sequence analysis. RESULTS: From a total of 108 blood cultures, 36 (33%) were identified as S. gallolyticus subsp. pasteurianus with the nucleotide sequence identities of partial sodA gene with the reference strains ranging from 98.1 to 100%. Of these, 25 (69.4%) contained erythromycin resistance genes and erm(B) was the most predominant gene (30.6%), followed by erm(T) (19.4%) and mef(A) (5.6%). In addition, erm(B) was also detected in combination with lnu(B) (8.3%), erm(T) and mef(A) (2.8%), and mef(A) and lnu(B) (2.8%). It was interesting to note that lnu(B) was detected for the first time in S. gallolyticus subsp. pasteurianus in this study. For Tetracycline resistance genes, tet(L) and tet(M) were detected at 13.9% and 11.1%, respectively. However, tet(M) in combination with tet(L) was detected most commonly at 69.4% and with tet(L) and tet(O) at 5.6%. CONCLUSIONS: A number of erythromycin and Tetracycline resistance genes were detected in S. gallolyticus subsp. pasteurianus strains circulating in Thailand.
In Vitro Activity of New Tetracycline Analogs Omadacycline and Eravacycline Against Drug-Resistant Clinical Isolates of Mycobacterium abscessus.[Pubmed:30962331]
Antimicrob Agents Chemother. 2019 Apr 8. pii: AAC.00470-19.
Tigecycline is used in multidrug regimens for salvage therapy of Mycobacterium abscessus infections but is often poorly tolerated and has no oral formulation. Here, we report similar in vitro activity of two newly approved Tetracycline analogs, omadacycline and eravacycline, against 28 drug-resistant clinical isolates of M. abscessus complex. Since omadacycline and eravacycline appear better tolerated than tigecycline and omadacycline is also formulated for oral dosing, they may represent new treatment options for M. abscessus infections.
The Ternary Heterostructures of BiOBr/Ultrathin g-C(3)N(4)/Black Phosphorous Quantum Dot Composites for Photodegradation of Tetracycline.[Pubmed:30961042]
Polymers (Basel). 2018 Oct 9;10(10). pii: polym10101118.
Herein, we synthesized BiOBr/ultrathin g-C(3)N(4)/ternary heterostructures modified with black phosphorous quantum dots using a simple water bath heating and sonication method. The ternary heterostructure was then used for the photocatalytic degradation of Tetracycline in visible light, with an efficiency as high as 92% after 3 h of irradiation. Thus, the photodegradation efficiency is greatly improved compared to that of ultrathin g-C(3)N(4), BiOBr, and black phosphorous quantum dots alone. The synthesized ternary heterostructure improves the charge separation efficiency, thus increasing the photodegradation efficiency. This work provides a new and efficient method for the degradation of antibiotics in the environment.
Tetracycline antibiotics as PI3K inhibitors in the Nrf2-mediated regulation of antioxidative stress in zebrafish larvae.[Pubmed:30959454]
Chemosphere. 2019 Apr 2;226:696-703.
Scientific concern about veterinary antibiotics (VAs) residues in the aquatic environment has increased in recent years. However, little is known about the underlying molecular mechanism of antioxidative stress caused by VAs in fish. In this study, zebrafish larvae were exposed to two representatives of VAs, chlorTetracycline (CTC) and oxyTetracycline (OTC), for 48h. The oxidative stress responses and possible molecular mechanism of action were investigated. The results showed that the activities of CAT, SOD and GPx were significantly inhibited and the contents of GSH and MDA increased after CTC exposure. Moreover, SOD and CAT activity were parallel to their mRNA and protein levels. Under OTC exposure, CAT and GST activity were inhibited, while GPx activity was induced, and MDA content decreased significantly. After treatment with CTC and OTC, glucose levels and Nrf2 mRNA and protein levels in zebrafish larvae were significantly downregulated. Further molecular docking and molecular dynamics simulations revealed that CTC and OTC are capable of docking into the binding pocket of zebrafish PI3K, an important molecule in the activation of Nrf2, and can form stable interactions through hydrogen bonds. The overall results indicated that CTC and OTC significantly induced oxidative stress responses in zebrafish larvae, and both CTC and OTC act as inhibitors of PI3K to inhibit the activation of the Nrf2/ARE signaling pathway, thus reducing the antioxidant capacity of fish.


