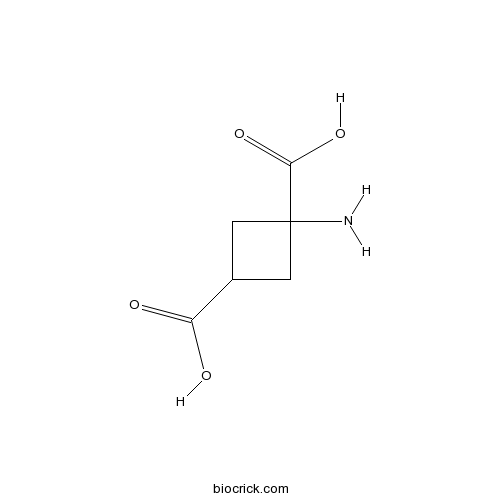
cis-ACBDPotent, selective L-glutamate uptake inhibitor CAS# 73550-55-7 |
- CFM 1571 hydrochloride
Catalog No.:BCC5924
CAS No.:1215548-30-3
- A 350619 hydrochloride
Catalog No.:BCC5939
CAS No.:1217201-17-6
- BAY 41-2272
Catalog No.:BCC7932
CAS No.:256376-24-6
- Riociguat
Catalog No.:BCC1899
CAS No.:625115-55-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 73550-55-7 | SDF | Download SDF |
| PubChem ID | 1214 | Appearance | Powder |
| Formula | C6H9NO4 | M.Wt | 159.14 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in 1eq. NaOH | ||
| Chemical Name | 1-aminocyclobutane-1,3-dicarboxylic acid | ||
| SMILES | C1C(CC1(C(=O)O)N)C(=O)O | ||
| Standard InChIKey | GGMYWPBNZXRMME-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C6H9NO4/c7-6(5(10)11)1-3(2-6)4(8)9/h3H,1-2,7H2,(H,8,9)(H,10,11) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, competitive and selective inhibitor of glutamate uptake. Certain confusion exists over the naming of this compound because of apparent contradictions in the literature. This is the isomer which has the carboxylic acid and the amino groups on the same side of the cyclobutyl ring. |

cis-ACBD Dilution Calculator

cis-ACBD Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.2838 mL | 31.4189 mL | 62.8378 mL | 125.6755 mL | 157.0944 mL |
| 5 mM | 1.2568 mL | 6.2838 mL | 12.5676 mL | 25.1351 mL | 31.4189 mL |
| 10 mM | 0.6284 mL | 3.1419 mL | 6.2838 mL | 12.5676 mL | 15.7094 mL |
| 50 mM | 0.1257 mL | 0.6284 mL | 1.2568 mL | 2.5135 mL | 3.1419 mL |
| 100 mM | 0.0628 mL | 0.3142 mL | 0.6284 mL | 1.2568 mL | 1.5709 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 7-Acetyllycopsamine
Catalog No.:BCN2000
CAS No.:73544-48-6
- Tetrachyrin
Catalog No.:BCN4776
CAS No.:73483-88-2
- 1-Deoxymannojirimycin hydrochloride
Catalog No.:BCC6995
CAS No.:73465-43-7
- Dehydrobruceine A
Catalog No.:BCN7620
CAS No.:73435-47-9
- Norandrostenedione
Catalog No.:BCC9103
CAS No.:734-32-7
- AR 231453
Catalog No.:BCC5143
CAS No.:733750-99-7
- SB 706375
Catalog No.:BCC6256
CAS No.:733734-61-7
- SCH 50911
Catalog No.:BCC5692
CAS No.:733717-87-8
- Niclosamide monohydrate
Catalog No.:BCC5212
CAS No.:73360-56-2
- 9-O-Acetyl-4,4'-di-O-methyllariciresinol
Catalog No.:BCN1367
CAS No.:73354-15-1
- Albanin A
Catalog No.:BCN3290
CAS No.:73343-42-7
- Macbecin I
Catalog No.:BCC7551
CAS No.:73341-72-7
- Mevastatin
Catalog No.:BCN2568
CAS No.:73573-88-3
- 27-p-Coumaroyloxyursolic acid
Catalog No.:BCN4288
CAS No.:73584-67-5
- Omeprazole
Catalog No.:BCC1254
CAS No.:73590-58-6
- (-)-Bicuculline methobromide
Catalog No.:BCC6555
CAS No.:73604-30-5
- 3-Hydroxybenzylamine
Catalog No.:BCN1804
CAS No.:73604-31-6
- Xylazine
Catalog No.:BCC5167
CAS No.:7361-61-7
- 1-(4-Methoxycinnamoyl)pyrrole
Catalog No.:BCN4027
CAS No.:736140-70-8
- 5-Amino-1-(2-hydroxyethyl)pyrazole
Catalog No.:BCC8727
CAS No.:73616-27-0
- p-Coumaric acid ethyl ester
Catalog No.:BCN4289
CAS No.:7362-39-2
- H-Asp-OBzl
Catalog No.:BCC2882
CAS No.:7362-93-8
- HEPES
Catalog No.:BCC7590
CAS No.:7365-45-9
- Naringenin 5-rhamnoside
Catalog No.:BCN2747
CAS No.:
Presynaptic excitability as a potential target for the treatment of the traumatic cerebellum.[Pubmed:15240995]
Pharmacology. 2004 Aug;71(4):192-8.
Using an extracellular recording method, we have previously shown a hyperexcitability of the presynaptic response in fluid percussion injury (FPI) in rats. In this study, we demonstrated that treatment with cis-ACBD, a glutamate reuptake inhibitor, depressed the presynaptic potential (PSP) in naive/sham controls, while it potentiated the PSP in FPI rats. On the contrary, (RS)-APICA, a selective group II metabotropic glutamate receptor antagonist, potentiated PSP in controls, but depressed PSP in FPI rats. These results indicate that an alteration of the normal function of metabotropic glutamate receptors and glutamate reuptake system or an altered reactivity of presynaptic fibers was induced by FPI. This alteration may contribute to the reported loss of Purkinje cells after FPI. PSP may be used as a potential tool for evaluating treatments of FPI or as a potential target for the prevention of Purkinje cell death.
L-trans-pyrrolidine-2,4-dicarboxylate and cis-1-aminocyclobutane-1,3-dicarboxylate behave as transportable, competitive inhibitors of the high-affinity glutamate transporters.[Pubmed:7905733]
Biochem Pharmacol. 1994 Jan 20;47(2):267-74.
The ability of two conformationally restricted analogues of L-glutamate to function as non-transportable inhibitors of plasma membrane L-glutamate transport was investigated in primary cultures of cerebellar granule cells and cortical astrocytes. L-trans-Pyrrolidine-2,4-dicarboxylic acid (L-trans-PDC) and cis-1-aminocyclobutane-1,3-dicarboxylic acid (cis-ACBD) behaved as linear competitive inhibitors of the uptake of D-[3H]aspartate (used as a non-metabolizable analogue of L-glutamate) exhibiting Ki values between 40 and 145 microM; L-trans-PDC being the more potent inhibitor in each preparation. However, both L-trans-PDC and cis-ACBD, over a concentration range of 1 microM-5 mM, dose-dependently stimulated the release of exogenously supplied D-[3H]aspartate from granule cells maintained in a continuous superfusion system. The stimulated release was independent of extracellular calcium ions; essentially superimposable dose-response profiles being obtained in the absence and presence of 1.3 mM CaCl2 and yielding EC50 values of 16-25 microM and 180-220 microM for L-trans-PDC and cis-ACBD, respectively. Stimulated release of D-[3H]aspartate was unaffected by either 300 microM D-(-)-2-amino-5-phosphonopentanoic acid [D-APV; a selective antagonist of the N-methyl-D-aspartate (NMDA) receptor] or by 25 microM 6-cyano-7-nitroquinoxaline-2,3-dione [CNQX; a selective antagonist of the alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptor]. The release of D-[3H]-aspartate following stimulation by either L-trans-PDC or cis-ACBD was however markedly attenuated following substitution in the superfusion medium of sodium ions by choline ions. Taken together, these results support an action of L-trans-PDC and cis-ACBD consistent with that of being competitive substrates rather than non-transportable blockers of the plasma membrane L-glutamate uptake system.
Differentiation of substrate and nonsubstrate inhibitors of the high-affinity, sodium-dependent glutamate transporters.[Pubmed:10570036]
Mol Pharmacol. 1999 Dec;56(6):1095-104.
Within the mammalian central nervous system, the efficient removal of L-glutamate from the extracellular space by excitatory amino acid transporters (EAATs) has been postulated to contribute to signal termination, the recycling of transmitter, and the maintenance of L-glutamate at concentrations below those that are excitotoxic. The development of potent and selective inhibitors of the EAATs has contributed greatly to the understanding of the functional roles of these transporters. In the present study, we use a library of conformationally constrained glutamate analogs to address two key issues: the differentiation of substrates from nontransportable inhibitors and the comparison of the pharmacological profile of synaptosomal uptake with those of the individual EAAT clones. We demonstrate that the process of transporter-mediated heteroexchange can be exploited in synaptosomes to rapidly distinguish transportable from nontransportable inhibitors. Using this approach, we demonstrate that 2,4-methanopyrrolidine-2,4-dicarboxylate, cis-1-aminocyclobutane-1,3-dicarboxylate, and L-trans-2, 4-pyrrolidine dicarboxylate act as substrates for the rat forebrain synaptosomal glutamate uptake system. In contrast, L-anti-endo-3, 4-methanopyrrolidine-3,4-dicarboxylate, L-trans-2,3-pyrrolidine dicarboxylate, and dihydrokainate proved to be competitive inhibitors of D-[(3)H]aspartate uptake that exhibited little or no activity as substrates. When these same compounds were characterized for substrate activity by recording currents in voltage-clamped Xenopus laevis oocytes expressing the human transporter clones EAAT1, EAAT2, or EAAT3, it was found that the pharmacological profile of the synaptosomal system exhibited the greatest similarity with the EAAT2 subtype, a transporter believed to be expressed primarily on glial cells.
Inhibition of high affinity L-glutamic acid uptake into rat cortical synaptosomes by the conformationally restricted analogue of glutamic acid, cis-1-aminocyclobutane-1,3-dicarboxylic acid.[Pubmed:1673544]
Neurosci Lett. 1991 Jan 2;121(1-2):133-5.
The action of two cyclobutane derivatives of L-glutamic acid on the high affinity uptake of L-glutamic acid was investigated using a preparation of synaptosomes from rat cerebral cortex. cis-1-Aminocyclobutane-1,3-dicarboxylic acid (also known as trans-2,4-methanoglutamic acid) potently inhibited L-glutamic acid uptake (IC50 30 microM), whereas trans-1-aminocyclobutane-1,3-dicarboxylic acid (also known as cis-2,4-methanoglutamic acid), a potent N-methyl-D-aspartate (NMDA) agonist, was inactive. Analysis of the kinetics of L-glutamic acid uptake in the presence and absence of cis-1-aminocyclobutane-1,3-dicarboxylic acid (CACB) suggests that it may act as a competitive inhibitor (Ki 8 microM). CACB may be substrate for the L-glutamic acid high-affinity uptake carrier since preincubation of CACB with the synaptosomal preparation increased its potency in inhibiting L-glutamic acid uptake. The conformationally restricted structure of CACB may be indicative of the conformations of L-glutamic acid that interact with the high affinity uptake carrier.
Synthesis and activity of a potent N-methyl-D-aspartic acid agonist, trans-1-aminocyclobutane-1,3-dicarboxylic acid, and related phosphonic and carboxylic acids.[Pubmed:2145435]
J Med Chem. 1990 Oct;33(10):2905-15.
We report the synthesis of a series of 3-carboxy-, 3-(carboxymethyl)-, 3-(omega-phosphonoalkyl)-1-aminocyclobutane-1-carboxylic acids for evaluation as agonists or antagonists of neurotransmission at excitatory amino acid receptors, particularly N-methyl-D-aspartic acid (NMDA) receptors. The compounds were evaluated as agonists on their ability to depolarize the rat brain cortical wedge preparation or as antagonist of the actions of the selective agonists NMDA, quisqualic acid, and kainic acid. The chain-elongated glutamate derivatives with potential antagonist activity proved to be weak and frequently nonselective antagonists in this assay. The most noteworthy result was that trans isomer 7b was a very potent agonist, approximately 20 times more active than NMDA at NMDA receptors, while the cis isomer was 1/3 as potent as NMDA.


