RiociguatSoluble guanylate cyclase (sGC) stimulator CAS# 625115-55-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 625115-55-1 | SDF | Download SDF |
| PubChem ID | 11304743 | Appearance | Powder |
| Formula | C20H19FN8O2 | M.Wt | 422.42 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BAY 632521 | ||
| Solubility | DMSO : ≥ 50 mg/mL (118.37 mM) *"≥" means soluble, but saturation unknown. | ||
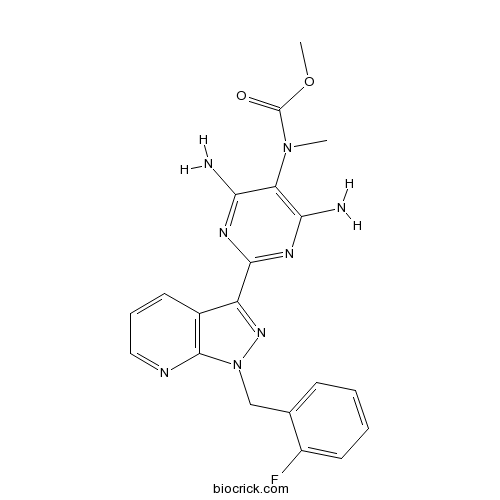
| Chemical Name | methyl N-[4,6-diamino-2-[1-[(2-fluorophenyl)methyl]pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-5-yl]-N-methylcarbamate | ||
| SMILES | CN(C1=C(N=C(N=C1N)C2=NN(C3=C2C=CC=N3)CC4=CC=CC=C4F)N)C(=O)OC | ||
| Standard InChIKey | WXXSNCNJFUAIDG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H19FN8O2/c1-28(20(30)31-2)15-16(22)25-18(26-17(15)23)14-12-7-5-9-24-19(12)29(27-14)10-11-6-3-4-8-13(11)21/h3-9H,10H2,1-2H3,(H4,22,23,25,26) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Riociguat is an oral stimulator of soluble guanylate cyclase (sGC), and used in the treatment of pulmonary hypertension.In Vitro:Riocigua stimulates the recombinant sGC concentration dependently from 0.1 to 100 μM with a two-fold to 73-fold effect by an NO-independent but haem-dependent mechanism[1]. Riociguat inhibits platelet function in washed platelets but not in whole blood, and exerts no direct effects on contractility and relaxation of cardiac myocytes[2].In Vivo:Riociguat (10 mg/kg/d, p.o.) partially reverses the pulmonary arterial hypertension, the right heart hypertrophy and the structural remodelling of the lung vasculature in chronic treatment of hypoxic mice and MCT-injected rats[1]. References: | |||||

Riociguat Dilution Calculator

Riociguat Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3673 mL | 11.8366 mL | 23.6731 mL | 47.3462 mL | 59.1828 mL |
| 5 mM | 0.4735 mL | 2.3673 mL | 4.7346 mL | 9.4692 mL | 11.8366 mL |
| 10 mM | 0.2367 mL | 1.1837 mL | 2.3673 mL | 4.7346 mL | 5.9183 mL |
| 50 mM | 0.0473 mL | 0.2367 mL | 0.4735 mL | 0.9469 mL | 1.1837 mL |
| 100 mM | 0.0237 mL | 0.1184 mL | 0.2367 mL | 0.4735 mL | 0.5918 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Riociguat (BAY 63-2521; BAY 632521) is a novel drug that is currently in clinical development by Bayer. Riociguat (BAY 63-2521; BAY 632521) is a stimulator of soluble guanylate cyclase (sGC). At the moment Phase III clinical trials investigate the use of riociguat as a new approach to treat two forms of pulmonary hypertension (PH): chronic thromboembolic pulmonary hypertension (CTEPH) and pulmonary arterial hypertension (PAH). Riociguat constitutes the first drug of a novel class of sGC stimulators.
- Viniferol D
Catalog No.:BCN4164
CAS No.:625096-18-6
- 3-Hydroxybutyric acid
Catalog No.:BCN2212
CAS No.:625-71-8
- 4-Amino-4-methyl-2-pentanone
Catalog No.:BCN1772
CAS No.:625-04-7
- Isodihydrofutoquinol B
Catalog No.:BCN6690
CAS No.:62499-71-2
- Parishin A
Catalog No.:BCN3811
CAS No.:62499-28-9
- Gastrodin
Catalog No.:BCN6306
CAS No.:62499-27-8
- 23-O-Acetylshengmanol 3-O-beta-D-xyloside
Catalog No.:BCN7947
CAS No.:62498-88-8
- 3-O-Acetyloleanderolide
Catalog No.:BCN4162
CAS No.:62498-83-3
- MK-0812
Catalog No.:BCC1755
CAS No.:624733-88-6
- p-Vinylphenyl O-beta-D-glucopyranoside
Catalog No.:BCN1393
CAS No.:62470-46-6
- Ursonic acid
Catalog No.:BCN4161
CAS No.:6246-46-4
- Dimethyl Fumarate
Catalog No.:BCC4776
CAS No.:624-49-7
- Ethyl p-hydroxyphenyllactate
Catalog No.:BCN6654
CAS No.:62517-34-4
- Tariquidar methanesulfonate, hydrate
Catalog No.:BCC1986
CAS No.:625375-83-9
- Isodihydrofutoquinol A
Catalog No.:BCN6691
CAS No.:62560-95-6
- H-D-Phe(4-Br)-OH
Catalog No.:BCC3158
CAS No.:62561-74-4
- Captopril
Catalog No.:BCC2140
CAS No.:62571-86-2
- 11R,12-Dihydroxyspirovetiv-1(10)-en-2-one
Catalog No.:BCN1392
CAS No.:62574-30-5
- Morusin
Catalog No.:BCN4165
CAS No.:62596-29-6
- Cyclomorusin
Catalog No.:BCN4610
CAS No.:62596-34-3
- Neocyclomorusin
Catalog No.:BCN3601
CAS No.:62596-35-4
- 2,4-Dihydroxypyridine
Catalog No.:BCC8500
CAS No.:626-03-9
- H-HoCys-OH
Catalog No.:BCC3231
CAS No.:626-72-2
- Oxiracetam
Catalog No.:BCC5447
CAS No.:62613-82-5
Haemodynamic effects of riociguat in inoperable/recurrent chronic thromboembolic pulmonary hypertension.[Pubmed:28011757]
Heart. 2017 Apr;103(8):599-606.
OBJECTIVE: We compared the haemodynamic effects of Riociguat in patients with inoperable chronic thromboembolic pulmonary hypertension (CTEPH) or persistent/recurrent CTEPH after pulmonary endarterectomy in the Chronic Thromboembolic Pulmonary Hypertension Soluble Guanylate Cyclase-Stimulator Trial 1 study. METHODS: Patients with inoperable or persistent/recurrent CTEPH (n=261; mean+/- SD age 59+/-14 years; 66% women) were randomised to Riociguat (up to 2.5 mg three times daily) or placebo. Haemodynamic parameters were assessed at baseline and week 16. RESULTS: Riociguat decreased pulmonary vascular resistance (PVR) in inoperable (n=189; least-squares mean difference: -285 dyn s/cm(5) (95% CI -357 to -213); p<0.0001) and persistent/recurrent (n=72; -131 dyn s/cm(5) (95% CI -214 to -48); p=0.0025) patients. Cardiac index improved in inoperable patients by a least-squares mean difference of +0.6 L/min/m(2) (95% CI 0.4 to 0.7; p<0.0001), while in persistent/recurrent patients the change was +0.2 L/min/m(2) (95% CI -0.1 to 0.5; p=0.17). Mean pulmonary artery pressure decreased in inoperable and persistent/recurrent patients(-4.7 mm Hg (95% CI -6.9 to -2.6; p<0.0001 and -4.8 mm Hg (-8.2 to -1.5; p=0.0055), respectively). For all patients, changes in 6 min walk distance correlated with changes in PVR (r=-0.29 (95% CI -0.41 to -0.17); p<0.0001) and cardiac index (r=0.23 (95% CI 0.10 to 0.35); p=0.0004). CONCLUSIONS: Riociguat improved haemodynamics in patients with inoperable CTEPH or persistent/recurrent CTEPH. TRIAL REGISTRATION NUMBER: NCT00855465.
Riociguat for the treatment of pulmonary hypertension: a safety evaluation.[Pubmed:27750459]
Expert Opin Drug Saf. 2016 Dec;15(12):1671-1677.
INTRODUCTION: The development of pulmonary hypertension (PH) has multifactorial underlying pathophysiological causes and can be classified into five groups. While three different classes of therapeutic drugs are licensed for the treatment of pulmonary arterial hypertension (PAH, WHO group 1), specific medical therapies are lacking for other forms of PH, such as PH due to left heart disease. In 2013 Riociguat, a first-in class soluble guanylate cyclase stimulator, has also become available for the treatment of PAH. Riociguat was further introduced as the first approved pharmacotherapy for the treatment of patients with chronic thromboembolic PH (WHO group 4, CTEPH). Despite these advances in therapeutic options for patients with PH, none of these agents have been approved for the treatment of PH due to left heart disease. Areas covered: We aim to give an overview of the pathophysiology of PH, pharmacodynamics and pharmacokinetic properties, safety and efficacy of Riociguat, including adverse events, contraindications and drug interactions. Expert opinion: Considering the increasingly broad indications for Riociguat in patients with PH, substantial knowledge of data and properties on safety and efficacy of Riociguat are becoming more and more important for physicians prescribing Riociguat to PH patients.
sGC stimulators: Evidence for riociguat beyond groups 1 and 4 pulmonary hypertension.[Pubmed:27890470]
Respir Med. 2017 Jan;122 Suppl 1:S28-S34.
Pulmonary hypertension (PH) is a chronic cardiopulmonary disorder that if left untreated, progresses rapidly and is ultimately fatal. The World Health Organization (WHO) has classified PH into 5 distinct groups according to pathophysiology, hemodynamic characteristics, and clinical presentation. Dysfunction in the nitric oxide (NO) pathway plays a key role in the pulmonary hypertension disease process, including in WHO Groups 2 and 3 PH. PH is associated with endothelial dysfunction, impaired synthesis of NO, and insufficient stimulation of the NO-soluble guanylate cyclase (sGC)-cyclic guanosine monophosphate (cGMP) pathway, which reduces cGMP production. cGMP regulates vascular tone, cellular proliferation, inflammation, and fibrosis and its depletion can lead to a variety of abnormalities, including pulmonary vasoconstriction, impaired vascular remodeling, and in situ thrombosis. This review will examine a novel class of drugs called sGC stimulators which directly stimulate sGC independently of NO, leading to increased production of cGMP.
Rationale and study design of RESPITE: An open-label, phase 3b study of riociguat in patients with pulmonary arterial hypertension who demonstrate an insufficient response to treatment with phosphodiesterase-5 inhibitors.[Pubmed:27887774]
Respir Med. 2017 Jan;122 Suppl 1:S18-S22.
Patients with pulmonary arterial hypertension (PAH) who do not have an adequate response to therapy with phosphodiesterase-5 inhibitors (PDE-5i) may have insufficient synthesis of cyclic guanosine monophosphate (cGMP). These patients may respond to a direct soluble guanylate cyclase (sGC) stimulator such as Riociguat. RESPITE (NCT02007629) was an open-label, multicenter, uncontrolled, single-arm phase 3b study of Riociguat in patients with PAH who demonstrated an insufficient response to treatment with PDE-5i. Insufficient response was defined as World Health Organization functional class (WHO FC) III despite PDE-5i therapy for at least 90 days; 6-min walk distance (6MWD) of 165-440 m, and right-heart catheterization showing mean pulmonary artery pressure >30 mmHg, cardiac index <3.0 L/min/m(2), and pulmonary vascular resistance >400 dyn s cm(-5). PAH patients with an insufficient response to stable doses of sildenafil or tadalafil-either as monotherapy or in combination with an endothelin receptor antagonist-for at least 90 days were switched to Riociguat for 24 weeks. Starting at 1.0 mg TID, the dose of Riociguat was increased during the 8-week titration phase in 0.5-mg increments in 2-week intervals if the patient had no signs or symptoms of hypotension. In the ensuing 16-week maintenance phase, Riociguat was continued at the optimal individual dose. All efficacy outcomes were exploratory and include change from baseline to 24 weeks in 6MWD, cardiac index, N-terminal pro-brain natriuretic peptide, WHO FC, and quality of life and the proportion of patients with clinical worsening. The following biomarkers were to be measured: cGMP, asymmetric dimethyl arginine, growth-differentiation factor-15, and ST2. Results from RESPITE will help to determine if PAH patients who do not respond to PDE-5i are likely to benefit from treatment with an sGC stimulator. The study may also identify biomarkers that are able to suggest which patients are more likely to respond to sGC stimulators.


