CyclomorusinCAS# 62596-34-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

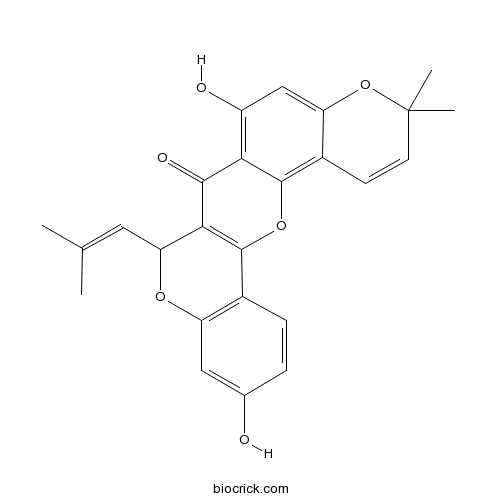
| Cas No. | 62596-34-3 | SDF | Download SDF |
| PubChem ID | 5481969 | Appearance | Yellow powder |
| Formula | C25H22O6 | M.Wt | 418.45 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CC(=CC1C2=C(C3=C(O1)C=C(C=C3)O)OC4=C5C=CC(OC5=CC(=C4C2=O)O)(C)C)C | ||
| Standard InChIKey | GDQXJMLXEYSICD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C25H22O6/c1-12(2)9-19-21-22(28)20-16(27)11-18-15(7-8-25(3,4)31-18)23(20)30-24(21)14-6-5-13(26)10-17(14)29-19/h5-11,19,26-27H,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Cyclomorusin , neoCyclomorusin and kuwanon C inhibited cholinesterase enzyme in a dose-dependent manner with K(i) values ranging between 3.1 and 37.5 uM and between 1.7 and 19.1 uM against acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) enzymes, respectively. 2. Cyclomorusin exhibits competitive inhibition toward monophenolase activity of mushroom tyrosinase, the IC50 value of 0.092 microM. 3. Cyclomorusin evokes the stimulation of superoxide anion generation in fMLP-stimulated rat neutrophils. |
| Targets | NO | NOS |

Cyclomorusin Dilution Calculator

Cyclomorusin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3898 mL | 11.9489 mL | 23.8977 mL | 47.7954 mL | 59.7443 mL |
| 5 mM | 0.478 mL | 2.3898 mL | 4.7795 mL | 9.5591 mL | 11.9489 mL |
| 10 mM | 0.239 mL | 1.1949 mL | 2.3898 mL | 4.7795 mL | 5.9744 mL |
| 50 mM | 0.0478 mL | 0.239 mL | 0.478 mL | 0.9559 mL | 1.1949 mL |
| 100 mM | 0.0239 mL | 0.1195 mL | 0.239 mL | 0.478 mL | 0.5974 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Morusin
Catalog No.:BCN4165
CAS No.:62596-29-6
- 11R,12-Dihydroxyspirovetiv-1(10)-en-2-one
Catalog No.:BCN1392
CAS No.:62574-30-5
- Captopril
Catalog No.:BCC2140
CAS No.:62571-86-2
- H-D-Phe(4-Br)-OH
Catalog No.:BCC3158
CAS No.:62561-74-4
- Isodihydrofutoquinol A
Catalog No.:BCN6691
CAS No.:62560-95-6
- Tariquidar methanesulfonate, hydrate
Catalog No.:BCC1986
CAS No.:625375-83-9
- Ethyl p-hydroxyphenyllactate
Catalog No.:BCN6654
CAS No.:62517-34-4
- Riociguat
Catalog No.:BCC1899
CAS No.:625115-55-1
- Viniferol D
Catalog No.:BCN4164
CAS No.:625096-18-6
- 3-Hydroxybutyric acid
Catalog No.:BCN2212
CAS No.:625-71-8
- 4-Amino-4-methyl-2-pentanone
Catalog No.:BCN1772
CAS No.:625-04-7
- Isodihydrofutoquinol B
Catalog No.:BCN6690
CAS No.:62499-71-2
- Neocyclomorusin
Catalog No.:BCN3601
CAS No.:62596-35-4
- 2,4-Dihydroxypyridine
Catalog No.:BCC8500
CAS No.:626-03-9
- H-HoCys-OH
Catalog No.:BCC3231
CAS No.:626-72-2
- Oxiracetam
Catalog No.:BCC5447
CAS No.:62613-82-5
- 2',4'-Dihydroxy-3'-methoxyacetophenone
Catalog No.:BCN7535
CAS No.:62615-26-3
- 4,6,7-Trimethoxy-5-methylcoumarin
Catalog No.:BCN4166
CAS No.:62615-63-8
- (+)-Mellein
Catalog No.:BCN7220
CAS No.:62623-84-1
- 11S,12-Dihydroxyspirovetiv-1(10)-en-2-one
Catalog No.:BCN1391
CAS No.:62623-86-3
- Ro 106-9920
Catalog No.:BCC7175
CAS No.:62645-28-7
- SIB 1893
Catalog No.:BCC6970
CAS No.:6266-99-5
- 1-Hydroxy-2-methylanthraquinone
Catalog No.:BCN3478
CAS No.:6268-09-3
- Handelin
Catalog No.:BCN2953
CAS No.:62687-22-3
Isolation of cholinesterase-inhibiting flavonoids from Morus lhou.[Pubmed:21434689]
J Agric Food Chem. 2011 May 11;59(9):4589-96.
Cholinesterases are key enzymes that play important roles in cholinergic transmission. Nine flavonoids displaying cholinesterase inhibitory activity were isolated from the root bark of Morus lhou L., a cultivated edible plant. The isolated compounds were identified as a new flavone (1), 5'-geranyl-5,7,2',4'-tetrahydroxyflavone (2), kuwanon U (3), kuwanon E (4), morusin (5), morusinol (6), Cyclomorusin (7), neoCyclomorusin (8), and kuwanon C (9). All compounds apart from compound 6 inhibited cholinesterase enzyme in a dose-dependent manner with K(i) values ranging between 3.1 and 37.5 muM and between 1.7 and 19.1 muM against acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) enzymes, respectively. The new compound was charactierized as 5'-geranyl-4'-methoxy-5,7,2'-trihydroxyflavone (1). It showed the most potent inhibitory activity (K(i) = 3.1 muM for AChE, K(i) = 1.74 muM for BChE). Lineweaver-Burk and Dixon plots and their secondary replots indicated that flavones (5-9) with prenyl substitution on C-3 were noncompetitive inhibitors, whereas those unsubstituted (1-4) at C-3 were mixed inhibitors of both AChE and BChE. In conclusion, this is the first study to demonstrate that alkylated flavonoids of M. lhou have potent inhibitory activities against AChE and BChE.
Antiinflammatory flavonoids from Artocarpus heterophyllus and Artocarpus communis.[Pubmed:15884809]
J Agric Food Chem. 2005 May 18;53(10):3867-71.
The antiinflammatory activities of the isolated flavonoids, including cycloartomunin (1), Cyclomorusin (2), dihydrocycloartomunin (3), dihydroisocycloartomunin (4), cudraflavone A (5), cyclocommunin (6), and artomunoxanthone (7), and cycloheterohyllin (8), artonins A (9) and B (10), artocarpanone (11), artocarpanone A (12), and heteroflavanones A (13), B (14), and C (15) from Artocarpus communis and A. heterophyllus, were assessed in vitro by determining their inhibitory effects on the chemical mediators released from mast cells, neutrophils, and macrophages. Compound 4 significantly inhibited the release of beta-glucuronidase and histamine from rat peritoneal mast cells stimulated with P-methoxy-N-methylphenethylamine (compound 48/80). Compound 11 significantly inhibited the release of lysozyme from rat neutrophils stimulated with formyl-Met-Leu-Phe (fMLP). Compounds 8, 10, and 11 significantly inhibited superoxide anion formation in fMLP-stimulated rat neutrophils while compounds 2, 3, 5, and 6 evoked the stimulation of superoxide anion generation. Compound 11 exhibited significant inhibitory effect on NO production and iNOS protein expression in RAW 264.7 cells. The potent inhibitory effect of compound 11 on NO production in lipopolysaccharide (LPS)-activated macrophages, probably through the suppression of iNOS protein expression.
Isoprenylated flavonoids from the root bark of Morus alba and their hepatoprotective and neuroprotective activities.[Pubmed:25981820]
Arch Pharm Res. 2015 Nov;38(11):2066-75.
A new isoprenylated flavonoid, 2S-5,7,2',4'-tetrahydroxy-3',5'-di-(gamma,gamma-dimethylallyl)flavanone, sanggenol Q (1), along with seven known isoprenylated flavonoids, sanggenol A (2), sanggenol L (3), kuwanon T (4), Cyclomorusin (5), sanggenon F (6), sanggenol O (7), and sanggenon N (8), three known Diels-Alder type adducts, sanggenon G (9), mulberrofuran G (10), and mulberrofuran C (11), and a known benzofuran, moracin E (12), were isolated from the root bark of Morus alba using silica gel, ODS, and Sephadex LH-20 column chromatography. Chemical structures were determined based on spectroscopic data analyses including NMR, MS, CD, and IR. For the first time, compounds 1 and 7 were isolated from the root bark of M. alba. All compounds were evaluated for hepatoprotective activity on t-BHP-induced oxidative stress in HepG2 cells and neuroprotective activity on glutamate-induced cell death in HT22 cells. Compounds 1, 4, 8, 10, and 11 showed protective effects on t-BHP-induced oxidative stress with EC50 values of 6.94 +/- 0.38, 30.32 +/- 6.82, 23.45 +/- 4.72, 15.31 +/- 2.21, and 0.41 +/- 0.48 muM, respectively, and compounds 1, 2, 10, 11, and 12 showed protective effects on glutamate-induced cell death with EC50 values of 5.54 +/- 0.86, 34.03 +/- 7.71, 19.71 +/- 0.71, 16.50 +/- 7.82, and 1.02 +/- 0.13 muM, respectively.
Inhibitory effects on mushroom tyrosinase by flavones from the stem barks of Morus lhou (S.) Koidz.[Pubmed:18608767]
J Enzyme Inhib Med Chem. 2008 Dec;23(6):922-30.
Five flavones displaying tyrosinase inhibitory activity were isolated from the stem barks of Morus lhou (S.) Koidz., a cultivated edible plant. The isolated compounds were identified as mormin (1), Cyclomorusin (2), morusin (3), kuwanon C (4), and norartocarpetin (5). Mormin (1) was characterized as a new flavone possesing a 3-hydroxymethyl-2-butenyl at C-3. The inhibitory potencies of these flavonoids toward monophenolase activity of mushroom tyrosinase were investigated. The IC50 values of compounds 1-5 for monophenolase activity were determined to be 0.088, 0.092, 0.250, 0.135 mM, and 1.2 microM, respectively. Mormin (1), Cyclomorusin (2), kuwanon C (4) and norartocarpetin (5) exhibited competitive inhibition characteristics. Interestingly norartocarpetin (5) showed a time-dependent inhibition against oxidation of L-tyrosine: it also operated under the enzyme isomerization model (k5 = 0.8424 min(-1), k6 = 0.0576 min(-1), K(app)(i) = 1.354 microM).


