NeocyclomorusinCAS# 62596-35-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 62596-35-4 | SDF | Download SDF |
| PubChem ID | 5481973 | Appearance | Yellow powder |
| Formula | C25H24O7 | M.Wt | 436.5 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
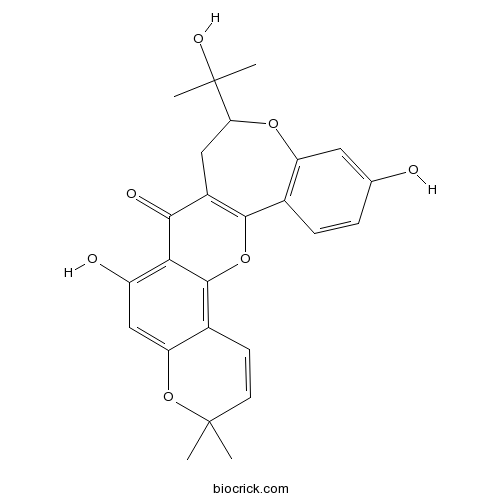
| SMILES | CC1(C=CC2=C3C(=C(C=C2O1)O)C(=O)C4=C(O3)C5=C(C=C(C=C5)O)OC(C4)C(C)(C)O)C | ||
| Standard InChIKey | BKIVBOLDWRIFMA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C25H24O7/c1-24(2)8-7-14-18(32-24)11-16(27)20-21(28)15-10-19(25(3,4)29)30-17-9-12(26)5-6-13(17)22(15)31-23(14)20/h5-9,11,19,26-27,29H,10H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Neocyclomorusin displays minimal inhibitory concentration (MIC) value of 4 ug/mL against Klebsiella pneumoniae ATCC11296, Enterobacter cloacae BM47. |
| Targets | Antifection |

Neocyclomorusin Dilution Calculator

Neocyclomorusin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.291 mL | 11.4548 mL | 22.9095 mL | 45.819 mL | 57.2738 mL |
| 5 mM | 0.4582 mL | 2.291 mL | 4.5819 mL | 9.1638 mL | 11.4548 mL |
| 10 mM | 0.2291 mL | 1.1455 mL | 2.291 mL | 4.5819 mL | 5.7274 mL |
| 50 mM | 0.0458 mL | 0.2291 mL | 0.4582 mL | 0.9164 mL | 1.1455 mL |
| 100 mM | 0.0229 mL | 0.1145 mL | 0.2291 mL | 0.4582 mL | 0.5727 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cyclomorusin
Catalog No.:BCN4610
CAS No.:62596-34-3
- Morusin
Catalog No.:BCN4165
CAS No.:62596-29-6
- 11R,12-Dihydroxyspirovetiv-1(10)-en-2-one
Catalog No.:BCN1392
CAS No.:62574-30-5
- Captopril
Catalog No.:BCC2140
CAS No.:62571-86-2
- H-D-Phe(4-Br)-OH
Catalog No.:BCC3158
CAS No.:62561-74-4
- Isodihydrofutoquinol A
Catalog No.:BCN6691
CAS No.:62560-95-6
- Tariquidar methanesulfonate, hydrate
Catalog No.:BCC1986
CAS No.:625375-83-9
- Ethyl p-hydroxyphenyllactate
Catalog No.:BCN6654
CAS No.:62517-34-4
- Riociguat
Catalog No.:BCC1899
CAS No.:625115-55-1
- Viniferol D
Catalog No.:BCN4164
CAS No.:625096-18-6
- 3-Hydroxybutyric acid
Catalog No.:BCN2212
CAS No.:625-71-8
- 4-Amino-4-methyl-2-pentanone
Catalog No.:BCN1772
CAS No.:625-04-7
- 2,4-Dihydroxypyridine
Catalog No.:BCC8500
CAS No.:626-03-9
- H-HoCys-OH
Catalog No.:BCC3231
CAS No.:626-72-2
- Oxiracetam
Catalog No.:BCC5447
CAS No.:62613-82-5
- 2',4'-Dihydroxy-3'-methoxyacetophenone
Catalog No.:BCN7535
CAS No.:62615-26-3
- 4,6,7-Trimethoxy-5-methylcoumarin
Catalog No.:BCN4166
CAS No.:62615-63-8
- (+)-Mellein
Catalog No.:BCN7220
CAS No.:62623-84-1
- 11S,12-Dihydroxyspirovetiv-1(10)-en-2-one
Catalog No.:BCN1391
CAS No.:62623-86-3
- Ro 106-9920
Catalog No.:BCC7175
CAS No.:62645-28-7
- SIB 1893
Catalog No.:BCC6970
CAS No.:6266-99-5
- 1-Hydroxy-2-methylanthraquinone
Catalog No.:BCN3478
CAS No.:6268-09-3
- Handelin
Catalog No.:BCN2953
CAS No.:62687-22-3
- Saikosaponin F
Catalog No.:BCN2776
CAS No.:62687-63-2
[Chemical constituents from Morus notabilis and their cytotoxic effect].[Pubmed:26234140]
Yao Xue Xue Bao. 2015 May;50(5):579-82.
Une new flavonoids named as notabilisin K (1), together with four known compounds, morusin (2), mulberrofuran A (3), Neocyclomorusin (4) and mornigrol F (5) are separated from 95% ethanol extracts of the twigs of Morus notabilis. Compounds 2-5 are separated from this plant for the first time. Notabilisin I, notabilisin J exhibits certain effect against cells of HCT-116, HepG2 and A2780 with IC50 values ranging from 1.47 mumol x L(-1) to 5.46 mumol x L(-1). Morusin exhibits strong effect against five kinds of human cancer cells (BGC823, A2780, HCT-116, HepG2 and NCI-H1650) with IC50 values ranging from 0.74 mumol x L(-1) to 1.58 mumol x L(-1).
Antibacterial activity of nineteen selected natural products against multi-drug resistant Gram-negative phenotypes.[Pubmed:26753111]
Springerplus. 2015 Dec 30;4:823.
The present study was designed to assess the antimicrobial activity of 19 natural products belonging to terpenoids, alkaloids, thiophenes and phenolics against a panel of 14 Gram-negative multidrug-resistant (MDR) bacteria. The results demonstrated that amongst the studied compounds, alkaloids and terpenoids were less active contrary to flavonoids: Neocyclomorusin (3) and candidone (6) and isoflavonoids: neobavaisoflavone (8) and daidzein (12). Thiophene, 2-(penta-1,3-diynyl)-5-(3,4-dihydroxybut-1-ynyl)thiophene (17) showed moderate and selective activities. Compounds 3, 6, 8 and 12 displayed minimal inhibitory concentration (MIC) ranged from 4 to 256 mug/mL on all the 14 tested bacteria. MIC values below 10 mug/mL were obtained with 8, 3, 6 and 12 against 50, 42.9, 35.7 and 21.4 % of the tested bacteria. The lowest MIC value of 4 mug/mL was obtained with compound 3 against Klebsiella pneumoniae ATCC11296, Enterobacter cloacae BM47, compound 6 against Escherichia coli ATCC8739, K. pneumoniae ATCC11296, E. cloacae BM47 and compound 8 against K. pneumoniae ATCC11296 and E. cloacae BM47. The activity of flavonoid 3 was better or equal to that of chloramphenicol in all tested K. pneumoniae, Providencia stuartii, E. aerogenes, E. cloacae and Pseudomonas aeruginosa strains. Within isoflavonoids, neobavaisoflavone scaffold was detected as a pharmacophoric moiety. This study indicates that natural products such as 3, 6 and 8 could be explored more to develop antimicrobial drugs to fight MDR bacterial infections.


