MorusinCAS# 62596-29-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 62596-29-6 | SDF | Download SDF |
| PubChem ID | 5281671 | Appearance | Beige-orange powder |
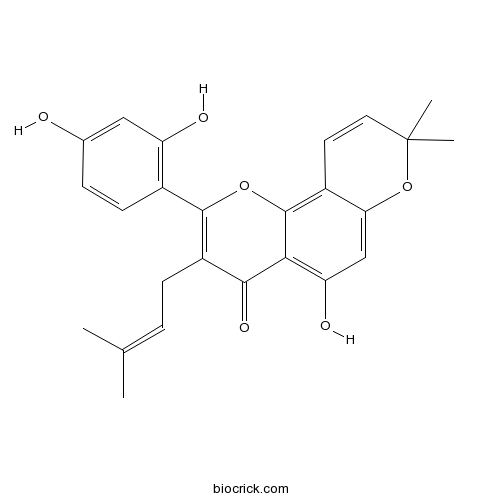
| Formula | C25H24O6 | M.Wt | 420.5 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Mulberrochromene | ||
| Solubility | DMSO : ≥ 125 mg/mL (297.30 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 2-(2,4-dihydroxyphenyl)-5-hydroxy-8,8-dimethyl-3-(3-methylbut-2-enyl)pyrano[2,3-h]chromen-4-one | ||
| SMILES | CC(=CCC1=C(OC2=C3C=CC(OC3=CC(=C2C1=O)O)(C)C)C4=C(C=C(C=C4)O)O)C | ||
| Standard InChIKey | XFFOMNJIDRDDLQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C25H24O6/c1-13(2)5-7-17-22(29)21-19(28)12-20-16(9-10-25(3,4)31-20)24(21)30-23(17)15-8-6-14(26)11-18(15)27/h5-6,8-12,26-28H,7H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Morusin exhibits antinociceptive, analgesic, anticonvulsant, antibacterial, and antitumor activities. Morusin can inhibit NF-κB and STAT3 activity, activate caspases activity, and restorate GABA level. |
| Targets | MMP(e.g.TIMP) | STAT | NF-kB | Bcl-2/Bax | Caspase | Antifection | GABA Receptor | PDK | PI3K | Akt | IkB | IKK |
| In vitro | Antitumor progression potential of morusin suppressing STAT3 and NFκB in human hepatoma SK-Hep1 cells.[Pubmed: 25476160]Toxicol Lett. 2015 Jan 22;232(2):490-8.Morusin is a prenylated flavonoid that has been isolated from the root bark of the mulberry tree (Morus species, Moraceae), a Chinese traditional medicine.
It has been synthesized by our laboratory from commercially available phloroglucinol, and has demonstrated to possess antitumor effects of cell lines including A549, MCF-7, and MDA-MB-231.
Morusin inhibits glioblastoma stem cell growth in vitro and in vivo through stemness attenuation, adipocyte transdifferentiation, and apoptosis induction.[Pubmed: 25557841]Mol Carcinog. 2014 Dec 31.Glioblastoma multiforme (GBM) cancer stem cells (GSCs) are responsible for the progression and recurrence of GBM after conventional therapy. Morusin possesses anti-cancer activity in vitro. The purpose of this study is to confirm the growth inhibition effect of Morusin on human GSCs growth in vitro and in vivo and to explore the possible mechanism of its activity.
An Investigation on Antibacterial Activity and Stability of Morusin.[Reference: WebLink]Science of Sericulture, 2013, 39(6):1150-4.
|
| In vivo | Antinociceptive properties of morusin, a prenylflavonoid isolated from Morus nigra root bark.[Pubmed: 10817216]Z Naturforsch C. 2000 Mar-Apr;55(3-4):256-60.The antinociceptive effects of Morusin (1), the main prenylflavonoid present in the Morus nigra root barks have been investigated in classical models of pain in mice.
|
| Kinase Assay | Morusin induces cell death through inactivating STAT3 signaling in prostate cancer cells.[Pubmed: 25628938]Am J Cancer Res. 2014 Dec 15;5(1):289-99.STAT3 has been recognized as an efficacious drug target for prostate cancer because of its constitutive activation in this fatal disease. We recently identified the root bark of Morus alba Linn. as a potential STAT3 inhibitor among 33 phytomedicines traditionally used in Korea. Morusin, an active compound isolated from the root bark of Morus alba, has shown anti-oxidant and anti-inflammatory effects.
In the present study, we examined whether Morusin has a potential as an anti-cancer agent in prostate cancer. |
| Cell Research | Morusin induces apoptosis and suppresses NF-kappaB activity in human colorectal cancer HT-29 cells.[Pubmed: 18485277 ]Biochem Biophys Res Commun. 2008 Jul 18;372(1):236-42.Morusin is a pure compound isolated from root bark of Morusaustralis (Moraceae). In this study, we Morusin is a pure compound isolated from root bark of Morusaustralis (Moraceae). In this study, we demonstrated that Morusin significantly inhibited the growth and clonogenicity of human colorectal cancer HT-29 cells.
|
| Animal Research | Anticonvulsant activity of Morusin isolated from : Modulation of GABA receptor.[Reference: WebLink]Biomed. Aging Pathol., 2013, 4(1):29–32.Epilepsy is a complex neurological disorder affecting 50 million of world's total population. Number of medicinal plants has been used to treat the convulsion. In ancient time Morus alba was used to treat epilepsy and mental illness. In Chinese medicine also M. alba is used as neuroprotective herbs. The present study was designed to explore the effect of Morusin, a flavonoid glycoside isolated from M. alba as anticonvulsant activity along with biochemical mechanism.
|

Morusin Dilution Calculator

Morusin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3781 mL | 11.8906 mL | 23.7812 mL | 47.5624 mL | 59.453 mL |
| 5 mM | 0.4756 mL | 2.3781 mL | 4.7562 mL | 9.5125 mL | 11.8906 mL |
| 10 mM | 0.2378 mL | 1.1891 mL | 2.3781 mL | 4.7562 mL | 5.9453 mL |
| 50 mM | 0.0476 mL | 0.2378 mL | 0.4756 mL | 0.9512 mL | 1.1891 mL |
| 100 mM | 0.0238 mL | 0.1189 mL | 0.2378 mL | 0.4756 mL | 0.5945 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Morusin is a prenylated flavonoid isolated from M. australis with various biological activities, such as antitumor, antioxidant, and anti-bacteria property. Morusin could inhibit NF-κB activity.
In Vitro:Morusin exhibits a dose- and time-dependent inhibitory effect on murine and human breast cancer cells. IC50 is 9.48 μg/mL for normal mammary epithelial cells (MCF-10A); 2.03 and 1.87 μg/mL for murine breast cancer cells (4 T1 and EMT6); and 2.71 and 3.86 μg/mL for human breast cancer cells (MCF-7 and MDA-MB-231), respectively, the maximal inhibition of cell growth (>80 %) is obtained at 8 μg/mL. The apoptotic cells in morusin treated breast cancer cells are increased significantly in a dose-dependent manner[1]. Morusin significantly inhibits the growth and clonogenicity of human colorectal cancer HT-29 cells. Morusin also inhibits the phosphorylation of IKK-α, IKK-βand IκB-β, increases expression of IκB-α, and suppresses nuclear translocation of NF-κB and its DNA binding activity. Dephosphorylation of NF-κB upstream regulators PI3K, Akt and PDK1 is also displayed. In addition, activation of caspase-8, change of mitochondrial membrane potential, release of cytochrome c and Smac/DIABLO, and activation of caspase-9 and -3 are observed at the early time point. Downregulation in the expression of Ku70 and XIAP is exhibited afterward[2]. Morusin suppresses viability of prostate cancer cells, but little effect in normal human prostate epithelial cells. Morusin also reduces STAT3 activity by inhibiting its phosphorylation, nuclear accumulation, and DNA binding activity. In addition, morusin down-regulated expression of STAT3 target genes encoding Bcl-xL, Bcl-2, Survivin, c-Myc and Cyclin D1. It induces apoptosis in human prostate cancer cells by reducing STAT3 activity[3].
In Vivo:Morusin retards the growth of breast cancer significantly. Mean tumor weight of the control mice is 1.14±0.30 g, and those of the mice administrated with 5 and 10 mg/kg of morusin are 0.61±0.23 and 0.41±0.10 g, respectively, tumor inhibitory rates are 46.5 %, and 64.1 %, respectively[1].
References:
[1]. Li H, et al. Morusin suppresses breast cancer cell growth in vitro and in vivo through C/EBPβ and PPARγ mediated lipoapoptosis. J Exp Clin Cancer Res. 2015 Nov 4;34:137.
[2]. Lee JC, et al. Morusin induces apoptosis and suppresses NF-kappaB activity in human colorectal cancer HT-29 cells. Biochem Biophys Res Commun. 2008 Jul 18;372(1):236-42.
[3]. Lim SL, et al. Morusin induces cell death through inactivating STAT3 signaling in prostate cancer cells. Am J Cancer Res. 2014 Dec 15;5(1):289-99.
- 11R,12-Dihydroxyspirovetiv-1(10)-en-2-one
Catalog No.:BCN1392
CAS No.:62574-30-5
- Captopril
Catalog No.:BCC2140
CAS No.:62571-86-2
- H-D-Phe(4-Br)-OH
Catalog No.:BCC3158
CAS No.:62561-74-4
- Isodihydrofutoquinol A
Catalog No.:BCN6691
CAS No.:62560-95-6
- Tariquidar methanesulfonate, hydrate
Catalog No.:BCC1986
CAS No.:625375-83-9
- Ethyl p-hydroxyphenyllactate
Catalog No.:BCN6654
CAS No.:62517-34-4
- Riociguat
Catalog No.:BCC1899
CAS No.:625115-55-1
- Viniferol D
Catalog No.:BCN4164
CAS No.:625096-18-6
- 3-Hydroxybutyric acid
Catalog No.:BCN2212
CAS No.:625-71-8
- 4-Amino-4-methyl-2-pentanone
Catalog No.:BCN1772
CAS No.:625-04-7
- Isodihydrofutoquinol B
Catalog No.:BCN6690
CAS No.:62499-71-2
- Parishin A
Catalog No.:BCN3811
CAS No.:62499-28-9
- Cyclomorusin
Catalog No.:BCN4610
CAS No.:62596-34-3
- Neocyclomorusin
Catalog No.:BCN3601
CAS No.:62596-35-4
- 2,4-Dihydroxypyridine
Catalog No.:BCC8500
CAS No.:626-03-9
- H-HoCys-OH
Catalog No.:BCC3231
CAS No.:626-72-2
- Oxiracetam
Catalog No.:BCC5447
CAS No.:62613-82-5
- 2',4'-Dihydroxy-3'-methoxyacetophenone
Catalog No.:BCN7535
CAS No.:62615-26-3
- 4,6,7-Trimethoxy-5-methylcoumarin
Catalog No.:BCN4166
CAS No.:62615-63-8
- (+)-Mellein
Catalog No.:BCN7220
CAS No.:62623-84-1
- 11S,12-Dihydroxyspirovetiv-1(10)-en-2-one
Catalog No.:BCN1391
CAS No.:62623-86-3
- Ro 106-9920
Catalog No.:BCC7175
CAS No.:62645-28-7
- SIB 1893
Catalog No.:BCC6970
CAS No.:6266-99-5
- 1-Hydroxy-2-methylanthraquinone
Catalog No.:BCN3478
CAS No.:6268-09-3
Antinociceptive properties of morusin, a prenylflavonoid isolated from Morus nigra root bark.[Pubmed:10817216]
Z Naturforsch C. 2000 Mar-Apr;55(3-4):256-60.
The antinociceptive effects of Morusin (1), the main prenylflavonoid present in the Morus nigra root barks have been investigated in classical models of pain in mice. The results showed that 1 exhibits a promising antinociceptive or analgesic profile by the intraperitoneal route, being more potent than some standard drugs used as reference. The mechanism by which the Morusin exerts antinociceptive activity still remains undetermined, but our results strongly suggest that it involves the participation of the opioid system.
Morusin inhibits glioblastoma stem cell growth in vitro and in vivo through stemness attenuation, adipocyte transdifferentiation, and apoptosis induction.[Pubmed:25557841]
Mol Carcinog. 2016 Jan;55(1):77-89.
Glioblastoma multiforme (GBM) cancer stem cells (GSCs) are responsible for the progression and recurrence of GBM after conventional therapy. Morusin possesses anti-cancer activity in vitro. The purpose of this study is to confirm the growth inhibition effect of Morusin on human GSCs growth in vitro and in vivo and to explore the possible mechanism of its activity. Human GSCs were enriched under nonadhesive culture system, and characterized through neurosphere formation, toluidine blue staining, immunofluorescence staining, Western blotting analysis of stemness markers of CD133, nestin, Sox2 and Oct4, and tumorigenecity in vivo; the growth inhibition effect of Morusin on human GSCs in vitro and in vivo were tested by cell cytotoxicity, neurosphere formation inhibition, adipogenic differentiation, apoptosis induction, and tumor growth inhibition in vivo assays. The potential molecular mechanisms underlying the growth inhibition effect of Morusin on GSCs in vitro and in vivo were investigated with Western blotting evaluation of stemness, adipogenic, and apoptotic proteins in Morusin treated GSCs and tumor tissues. GSCs enriched under nonadhesive culture system possess stemness characterstics; Morusin inhibited GSCs growth in vitro and in vivo, it reduced stemness of GSCs, induced them adipocyte-like transdifferention and apoptosis. Morusin has the potential to inhibit human GSCs growth in vitro and in vivo through stemness attenuation, adipocyte transdifferentiation, and apoptosis induction.
Antitumor progression potential of morusin suppressing STAT3 and NFkappaB in human hepatoma SK-Hep1 cells.[Pubmed:25476160]
Toxicol Lett. 2015 Jan 22;232(2):490-8.
Morusin is a prenylated flavonoid that has been isolated from the root bark of the mulberry tree (Morus species, Moraceae), a Chinese traditional medicine. It has been synthesized by our laboratory from commercially available phloroglucinol, and has demonstrated to possess antitumor effects of cell lines including A549, MCF-7, and MDA-MB-231. In this study, at non-cytotoxic concentrations, Morusin altered invasive morphology and suppressed cell-matrix adhesion, cell motility and cell invasion in SK-Hep1 cells. Morusin also increased the expression of E-cadherin, an epithelial cell junction protein, decreased the expression of vimentin, a mesecnchymal marker, and alpha2-, alpha6-, beta1- integrin, which regulated cancer attachment and migration. In addition, Morusin reduced the activity of matrix metalloproteinase-2 and 9 (MMP-2 and MMP-9), which were involved in extracellular matrix (ECM) degradation and promoting cancer cell invasion. Furthermore, Morusin suppressed the signal transducer and activator of transcription 3 (STAT3) and nuclear factor-kappaB (NFkappaB) signaling pathways, which modulate the protein expression involved in the invasion process. Finally, Morusin decreased the lung colonization of the SK-Hep1 cells in the nude mice. These results indicate Morusin possesses antitumor progression potential through suppressing STAT3 and NFkappaB.
Morusin induces cell death through inactivating STAT3 signaling in prostate cancer cells.[Pubmed:25628938]
Am J Cancer Res. 2014 Dec 15;5(1):289-99. eCollection 2015.
STAT3 has been recognized as an efficacious drug target for prostate cancer because of its constitutive activation in this fatal disease. We recently identified the root bark of Morus alba Linn. as a potential STAT3 inhibitor among 33 phytomedicines traditionally used in Korea. Morusin, an active compound isolated from the root bark of Morus alba, has shown anti-oxidant and anti-inflammatory effects. In the present study, we examined whether Morusin has a potential as an anti-cancer agent in prostate cancer. We found that Morusin suppressed viability of prostate cancer cells, but little effect in normal human prostate epithelial cells. Morusin also reduced STAT3 activity by inhibiting its phosphorylation, nuclear accumulation, and DNA binding activity. In addition, Morusin down-regulated expression of STAT3 target genes encoding Bcl-xL, Bcl-2, Survivin, c-Myc and Cyclin D1, which are involved in regulation of apoptosis and cell cycle. Furthermore, Morusin induced apoptosis in human prostate cancer cells by reducing STAT3 activity. Taken together, these results suggest that Morusin could be a potentially therapeutic agent for prostate cancer by reducing STAT3 activity and inducing apoptosis.
Morusin induces apoptosis and suppresses NF-kappaB activity in human colorectal cancer HT-29 cells.[Pubmed:18485277]
Biochem Biophys Res Commun. 2008 Jul 18;372(1):236-42.
Morusin is a pure compound isolated from root bark of Morusaustralis (Moraceae). In this study, we demonstrated that Morusin significantly inhibited the growth and clonogenicity of human colorectal cancer HT-29 cells. Apoptosis induced by Morusin was characterized by accumulation of cells at the sub-G(1) phase, fragmentation of DNA, and condensation of chromatin. Morusin also inhibited the phosphorylation of IKK-alpha, IKK-beta and IkappaB-alpha, increased expression of IkappaB-alpha, and suppressed nuclear translocation of NF-kappaB and its DNA binding activity. Dephosphorylation of NF-kappaB upstream regulators PI3K, Akt and PDK1 was also displayed. In addition, activation of caspase-8, change of mitochondrial membrane potential, release of cytochrome c and Smac/DIABLO, and activation of caspase-9 and -3 were observed at the early time point. Downregulation in the expression of Ku70 and XIAP was exhibited afterward. Caspase-8 or wide-ranging caspase inhibitor suppressed Morusin-induced apoptosis. Therefore, the antitumor mechanism of Morusin in HT-29 cells may be via activation of caspases and inhibition of NF-kappaB.


