Parishin ACAS# 62499-28-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 62499-28-9 | SDF | Download SDF |
| PubChem ID | 10557926 | Appearance | White powder |
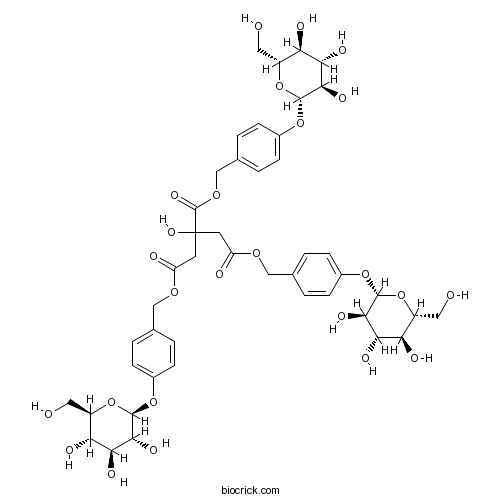
| Formula | C45H56O25 | M.Wt | 996.9 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in methan | ||
| Chemical Name | tris[[4-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]methyl] 2-hydroxypropane-1,2,3-tricarboxylate | ||
| SMILES | C1=CC(=CC=C1COC(=O)CC(CC(=O)OCC2=CC=C(C=C2)OC3C(C(C(C(O3)CO)O)O)O)(C(=O)OCC4=CC=C(C=C4)OC5C(C(C(C(O5)CO)O)O)O)O)OC6C(C(C(C(O6)CO)O)O)O | ||
| Standard InChIKey | WYKQPGOKTKQHQG-SHGJSZTHSA-N | ||
| Standard InChI | InChI=1S/C45H56O25/c46-15-27-32(51)35(54)38(57)41(68-27)65-24-7-1-21(2-8-24)18-62-30(49)13-45(61,44(60)64-20-23-5-11-26(12-6-23)67-43-40(59)37(56)34(53)29(17-48)70-43)14-31(50)63-19-22-3-9-25(10-4-22)66-42-39(58)36(55)33(52)28(16-47)69-42/h1-12,27-29,32-43,46-48,51-59,61H,13-20H2/t27-,28-,29-,32-,33-,34-,35+,36+,37+,38-,39-,40-,41-,42-,43-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Parishin A has good neuroprotective effects against brain disorders. |
| Structure Identification | Chinese Journal of Experimental Traditional Medical Formulae, 2016, (18) :5-8.Comparison on Chemical Constituents and Pharmacological Effect of Gastrodiae Rhizoma Between Traditional Processing and Integration Processing[Reference: WebLink]To elucidate the rationality of integration processing technology based on comparison on chemical constituents and pharmacological effect of Gastrodiae Rhizoma between traditional processing and integration processing. |

Parishin A Dilution Calculator

Parishin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.0031 mL | 5.0155 mL | 10.0311 mL | 20.0622 mL | 25.0777 mL |
| 5 mM | 0.2006 mL | 1.0031 mL | 2.0062 mL | 4.0124 mL | 5.0155 mL |
| 10 mM | 0.1003 mL | 0.5016 mL | 1.0031 mL | 2.0062 mL | 2.5078 mL |
| 50 mM | 0.0201 mL | 0.1003 mL | 0.2006 mL | 0.4012 mL | 0.5016 mL |
| 100 mM | 0.01 mL | 0.0502 mL | 0.1003 mL | 0.2006 mL | 0.2508 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Gastrodin
Catalog No.:BCN6306
CAS No.:62499-27-8
- 23-O-Acetylshengmanol 3-O-beta-D-xyloside
Catalog No.:BCN7947
CAS No.:62498-88-8
- 3-O-Acetyloleanderolide
Catalog No.:BCN4162
CAS No.:62498-83-3
- MK-0812
Catalog No.:BCC1755
CAS No.:624733-88-6
- p-Vinylphenyl O-beta-D-glucopyranoside
Catalog No.:BCN1393
CAS No.:62470-46-6
- Ursonic acid
Catalog No.:BCN4161
CAS No.:6246-46-4
- Dimethyl Fumarate
Catalog No.:BCC4776
CAS No.:624-49-7
- Methyl levulinate
Catalog No.:BCN4160
CAS No.:624-45-3
- Cinnzeylanol
Catalog No.:BCN4159
CAS No.:62394-04-1
- Alboctalol
Catalog No.:BCN4158
CAS No.:62394-00-7
- Kadsuric acid
Catalog No.:BCN4157
CAS No.:62393-88-8
- Arjunglucoside II
Catalog No.:BCN6395
CAS No.:62369-72-6
- Isodihydrofutoquinol B
Catalog No.:BCN6690
CAS No.:62499-71-2
- 4-Amino-4-methyl-2-pentanone
Catalog No.:BCN1772
CAS No.:625-04-7
- 3-Hydroxybutyric acid
Catalog No.:BCN2212
CAS No.:625-71-8
- Viniferol D
Catalog No.:BCN4164
CAS No.:625096-18-6
- Riociguat
Catalog No.:BCC1899
CAS No.:625115-55-1
- Ethyl p-hydroxyphenyllactate
Catalog No.:BCN6654
CAS No.:62517-34-4
- Tariquidar methanesulfonate, hydrate
Catalog No.:BCC1986
CAS No.:625375-83-9
- Isodihydrofutoquinol A
Catalog No.:BCN6691
CAS No.:62560-95-6
- H-D-Phe(4-Br)-OH
Catalog No.:BCC3158
CAS No.:62561-74-4
- Captopril
Catalog No.:BCC2140
CAS No.:62571-86-2
- 11R,12-Dihydroxyspirovetiv-1(10)-en-2-one
Catalog No.:BCN1392
CAS No.:62574-30-5
- Morusin
Catalog No.:BCN4165
CAS No.:62596-29-6
Using 3D-UPLC-DAD and a new method-verification by adding mixture standard compounds to determine the fingerprint and eight active components of Naoluoxintong decoction.[Pubmed:30836247]
J Pharm Biomed Anal. 2019 May 30;169:60-69.
Naoluoxintong decoction (NLXTD) is a traditional Chinese formula which has been used for the management of ischemic stroke in China for two hundred years. In this study, we developed a comprehensive and reliable analytical method to qualitatively analyze the components in NLXTD. This novel method was based on three-dimensional ultra-fast high performance liquid chromatography coupled with diode array detector (3D-UPLC-DAD) with an additional component validation method via incorporation of the mixture standard compounds during the verification step. In addition, the relationship between active components and "Monarch drug, Minster drug, Assistant drug, Guide drug" were determined. Our results showed that gradient elution with the mobile phase of 0.02% formic acid and methanol was the optimum condition to separate peaks. A total of 35 common peaks were established by comparing ten batches of NLXTD, and eight components were identified, including Calycosin, Calycosin-7-O-beta-d-glucoside and Ononin in Astragali radix (Monarch drug); Ligustrazine in Chuanxiong Rhizoma (Minster drug); 4-Hydroxbenzyl alcohol and Parishin A in Gastrodiae rhizome (Assistant drug); Ferulic acid in Angelicae sinensis radix (Guide drug). The validation method of verification by adding mixture standard compounds combined with 3D-UPLC-DAD method, with the merits of greater resolution, higher speed of analysis and higher sensitivity, provided a semi-quantitative and qualitative analysis method to assess traditional Chinese medicinal prescription consisting of many bio-active components. Finally, our study has provided systemic and scientific evidence to explain the relationship between the bio-active components in the NLXTD and traditional Chinese medicine theory.
Comparison of Bioactive Compounds and Antioxidant Activities of Maclura tricuspidata Fruit Extracts at Different Maturity Stages.[Pubmed:30720740]
Molecules. 2019 Feb 4;24(3). pii: molecules24030567.
Abstract: Maclura tricuspidata fruit contains various bioactive compounds and has traditionally been used in folk medicine and as valuable food material in Korea. The composition and contents of bioactive compounds in the fruit can be influenced by its maturity stages. In this study, total phenol, total flavonoid, individual polyphenolic compounds, total carotenoids and antioxidant activities at four maturity stages of the fruit were determined. Polyphenolic compounds were analyzed using high-pressure liquid chromatography-quadrupole time-of-flight mass spectrometry (HPLC-QTOF-MS) and HPLC. Among 18 polyphenolic compounds identified in this study, five parishin derivatives (gastrodin, Parishin A, B, C, E) were positively identified for the first time in this plant. These compounds were also validated and quantified using authentic standards. Parishin A was the most abundant component, followed by chlorogenic acid, gastrodin, eriodictyol glucoside, parishin C, parishin E and parishin B. The contents of all the polyphenolic compounds were higher at the immature and premature stages than at fully mature and overmature stages, while total carotenoid was found to be higher in the mature and overmature stages. Overall antioxidant activities by three different assays (DPPH, ABTS, FRAP) decreased as maturation progressed. Antioxidant properties of the fruit extract are suggested to be attributed to the polyphenols.
Optimal Extraction Study of Gastrodin-Type Components from Gastrodia Elata Tubers by Response Surface Design with Integrated Phytochemical and Bioactivity Evaluation.[Pubmed:30717352]
Molecules. 2019 Feb 2;24(3). pii: molecules24030547.
Gastrodia elata tuber (GET) is a popular traditional Chinese medicines (TCMs). In this study, response surface methodology (RSM) with a Box(-)Behnken design (BBD) was performed to optimize the extraction parameters of gastrodin-type components (gastrodin, gastrodigenin, Parishin A, parishin B, parishin C and parishin E). Different from the conventional studies that merely focused on the contents of phytochemical, we gave consideration to both quantitative analysis of the above six components by HPLC and representative bioactivities of GET, including antioxidation and protection of human umbilical vein endothelial cells (HUVEC). Four independent variables (ethanol concentration, liquid-material ratio, soaking time and extraction time) were investigated with the integrated evaluation index of phytochemical contents. With the validation experiments, the optimal extraction parameters were as follows: ethanol concentration of 41%, liquid(-)solid ratio of 28.58 mL/g, soaking time of 23.91 h and extraction time of 46.60 min. Under the optimum conditions, the actual standardized comprehensive score was 1.8134 +/- 0.0110, which was in accordance with the predicted score of 1.8100. This firstly established method was proved to be feasible and reliable to optimize the extraction parameters of the bioactive components from GET. Furthermore, it provides some reference for the quality control and extraction optimization of TCMs.
Effect of inorganic salt on partition of high-polarity parishins in two-phase solvent systems and separation by high-speed counter-current chromatography from Gastrodia elata Blume.[Pubmed:30580477]
J Sep Sci. 2019 Feb;42(4):871-877.
Parishins are high-polarity and major bioactive constituents in Gastrodia elata Blume. In this study, the effect of several inorganic salts on the partition of parishins in two-phase solvent systems was investigated. Adding ammonium sulfate, which has a higher solubility in water, was found to significantly promote the partition of parishins in the upper organic polar solvents. Based on the results, a two-phase solvent system composed of butyl alcohol/acetonitrile/near-saturated ammonium sulfate solution/water (1.5:0.5:1.2:1, v/v/v/v) was used for the purification of parishins by high-speed counter-current chromatography. Fractions obtained from high-speed counter-current chromatography were subjected to semi-preparative high-performance liquid chromatography to remove salt and impurities. As a result, parishin E (6.0 mg), parishin B (7.8 mg), parishin C (3.2 mg), gastrodin (15.3 mg), and Parishin A (7.3 mg) were isolated from water extract of Gastrodia elata Blume (400 mg). These results demonstrated that adding inorganic salt that has high solubility in water to the two-phase solvent system in high-speed counter-current chromatography was a suitable approach for the purification of high-polarity compounds.
An Optimized and Sensitive Pharmacokinetic Quantitative Method of Investigating Gastrodin, Parishin, and Parishin B, C and E in Beagle Dog Plasma using LC-MS/MS after Intragastric Administration of Tall Gastrodia Capsules.[Pubmed:29125575]
Molecules. 2017 Nov 10;22(11). pii: molecules22111938.
Gastrodia elata Blume, called Tianma in China, has been widely used to treat headaches, convulsions and epilepsy for thousands of years. In the present study, a series of optimizations were employed to develop a rapid, sensitive, and reliable high-performance liquid chromatography-triple quadrupole mass spectrometry method, which was then used for the simultaneous determination of gastrodin, parishin, parishin B, parishin C and parishin E in beagle dog plasma after intragastric administration of tall Gastrodia capsules (Tianma brand). The chromatographic separation was achieved on a C18 column with gradient elution by using a mixture of 0.4% formic acid aqueous solution and acetonitrile as the mobile phase at a flow rate of 0.15 mL/min. A tandem mass spectrometric detection was conducted using multiple-reaction monitoring (MRM) via electrospray ionization (ESI) source in negative ionization mode. Samples were pre-treated by a single-step protein precipitation with methanol, and bergenin was used as internal standard (IS). Under the optimized conditions, the lower limit of quantification (LLOQ) was 0.10 ng/mL for gastrodin, 0.40 ng/mL for parishin B, 0.02 ng/mL for parishin E and 0.20 ng/mL for Parishin And parishin C, all of which previously were the highest levels of sensitivity. The methods were optimized for selectivity, calibration curves, accuracy and precision. Extraction recoveries, matrix effects and stability were within acceptable ranges. Pharmacokinetic parameters of the tested substances were also quantitatively determined. Finally, a possible metabolic pathway was induced based on correlations obtained from quantitative and qualitative data analysis in vivo.
[Development of Tianma HPLC fingerprint and discriminant analysis].[Pubmed:28840694]
Zhongguo Zhong Yao Za Zhi. 2017 Jul;42(13):2524-2531.
Tianma(the tuber of Gastrodia eleta) is a widely used and pricy Chinese herb. Its counterfeits are often found in herbal markets, which are the plant materials with similar macroscopic characteristics of Tianma. Moreover, the prices of Winter Tianma(cultivated Tianma) and Spring Tianma(mostly wild Tianma) have significant difference. However, it is difficult to identify the true or false, good or bad quality of Tianma samples. Thus, a total of 48 Tianma samples with different characteristics(including Winter Tianma, Spring Tianma, slice, powder, etc.) and 9 plant species 10 samples of Tianma counterfeits were collected and analyzed by HPLC-DAD-MS techniques. After optimizing the procedure of sample preparation, chromatographic and mass-spectral conditions, the HPLC chromatograms of all those samples were collected and compared. The similarities and Fisher discriminant analysis were further conducted between the HPLC chromatograms of Tianma and counterfeit, Winter Tianma and Spring Tianma. The results showed the HPLC chromatograms of 48 Tianma samples were similar at the correlation coefficient more than 0.848(n=48). Their mean chromatogram was simulated and used as Tianma HPLC fingerprint. There were 11 common peaks on the HPLC chromatograms of Tianma, in which 6 main peaks were chosen as characteristic peaks and identified as gastrodin, p-hydroxybenzyl alcohol, Parishin A, parishin B, parishin C, parishin E, respectively by comparison of the retention time, UV and MS data with those of standard chemical compounds. All the six chemical compounds are bioactive in Tianma. However, the HPLC chromatograms of the 10 counterfeit samples were significantly different from Tianma fingerprint. The correlation coefficients between HPLC fingerprints of Tianma with the HPLC chromatograms of counterfeits were less than 0.042 and the characteristic peaks were not observed on the HPLC chromatograms of these counterfeit samples. It indicated the true or false Tianma can be identified by either the similarity or characteristic peaks on HPLC fingerprint. Comparing the Winter Tianma with Spring Tianma showed that the HPLC chromatograms of 15 winter Tianma samples and 11 spring Tianma samples were similar at the mean correlation coefficient of 0.908. But the intensity of the characteristic peaks were different between the two groups of Tianma samples, i.e. the intensity of gastrodin, paishin A and C in winter Tianma was lower than those in spring Tianma. The Winter Tianma and Spring Tianma could be discriminated by either the Fisher unstandardized discrimination function or Linear discriminant function, based on the peak areas of 11 common peaks on HPLC chromatograms as variate.
[Structure-activity relationship of gastrodin and parishins on learning and memory deficits induced by scopolamine].[Pubmed:29874020]
Yao Xue Xue Bao. 2016 May;51(5):743-8.
Gastrodin, Parishin And parishin C were purified from a water extract of GE (rhizome of Gastrodia elata, an herb medicine for treatment of neuronal disorders). In order to compare the pharmacological effects of gastrodin, Parishin And parishin C on improving cognition deficits, we tested them in an animal model of cognition disorders induced by scopolamine and in a study of in vivo long-term potentiation (LTP) recordings. In the Morris water maze task, parishin C (15 and 50 mg.kg(-1), P<0.05) and parishin (150 mg.kg(-1), P<0.05), improved spatial learning and memory significantly. However, gastrodin showed no significant effects at the dose of 150 mg.kg(-1). In vivo LTP recordings showed that parishin C at 5,10 and 20 mg.kg(-1), Parishin At 10, 30 and 100 mg.kg(-1) reversed the suppression of LTP by scopolamine in rats in a dose-dependent manner. However, gastrodin at 100 mg.kg(-1) showed only a modest effect. In summary, the action of parishin C in the improvement of dementia induced by scopolamine was more potent than Parishin And gastrodin.


