Dimethyl FumarateNrf2 pathway activator; neuroprotective CAS# 624-49-7 |
- MLN9708
Catalog No.:BCC2091
CAS No.:1201902-80-8
- MG-115
Catalog No.:BCC1237
CAS No.:133407-86-0
- Clasto-Lactacystin β-lactone
Catalog No.:BCC1224
CAS No.:154226-60-5
- CEP-18770
Catalog No.:BCC2093
CAS No.:847499-27-8
- Carfilzomib (PR-171)
Catalog No.:BCC1145
CAS No.:868540-17-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 624-49-7 | SDF | Download SDF |
| PubChem ID | 637568 | Appearance | Powder |
| Formula | C6H8O4 | M.Wt | 144.13 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Dimethyl fumarate | ||
| Solubility | DMSO : 50 mg/mL (346.91 mM; Need ultrasonic) | ||
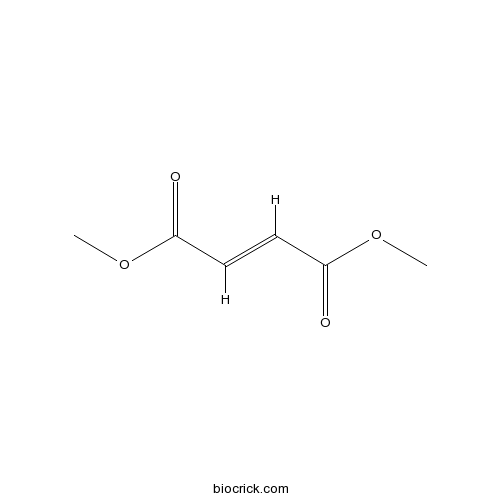
| Chemical Name | dimethyl (E)-but-2-enedioate | ||
| SMILES | COC(=O)C=CC(=O)OC | ||
| Standard InChIKey | LDCRTTXIJACKKU-ONEGZZNKSA-N | ||
| Standard InChI | InChI=1S/C6H8O4/c1-9-5(7)3-4-6(8)10-2/h3-4H,1-2H3/b4-3+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Nuclear factor (erythroid-derived)-like 2 (Nrf2) pathway activator; induces upregulation of antioxidant gene expression. Displays cytoprotective effects in human spinal cord astrocytes, oligodendrocyte precursor cells and hippocampal neurons following hydrogen peroxide challenge. Exerts neuroprotective effects in vivo in experimental autoimmune encephalomyelitis. Primary metabolite MMF available. |

Dimethyl Fumarate Dilution Calculator

Dimethyl Fumarate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.9382 mL | 34.6909 mL | 69.3818 mL | 138.7636 mL | 173.4545 mL |
| 5 mM | 1.3876 mL | 6.9382 mL | 13.8764 mL | 27.7527 mL | 34.6909 mL |
| 10 mM | 0.6938 mL | 3.4691 mL | 6.9382 mL | 13.8764 mL | 17.3455 mL |
| 50 mM | 0.1388 mL | 0.6938 mL | 1.3876 mL | 2.7753 mL | 3.4691 mL |
| 100 mM | 0.0694 mL | 0.3469 mL | 0.6938 mL | 1.3876 mL | 1.7345 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Dimethyl fumarate is a nuclear factor (erythroid-derived)-like 2 (Nrf2) pathway activator. Dimethyl fumarate induces upregulation of antioxidant gene expression.
- Methyl levulinate
Catalog No.:BCN4160
CAS No.:624-45-3
- Cinnzeylanol
Catalog No.:BCN4159
CAS No.:62394-04-1
- Alboctalol
Catalog No.:BCN4158
CAS No.:62394-00-7
- Kadsuric acid
Catalog No.:BCN4157
CAS No.:62393-88-8
- Arjunglucoside II
Catalog No.:BCN6395
CAS No.:62369-72-6
- Isotschimgin
Catalog No.:BCN4156
CAS No.:62356-47-2
- Oxyntomodulin
Catalog No.:BCC5874
CAS No.:62340-29-8
- H-Glu(OtBu)-OMe.HCl
Catalog No.:BCC2935
CAS No.:6234-01-1
- Lappaol A
Catalog No.:BCN8280
CAS No.:62333-08-8
- Arjunglucoside I
Catalog No.:BCN8259
CAS No.:62319-70-4
- Ki20227
Catalog No.:BCC1678
CAS No.:623142-96-1
- 5-Hydroxy-2-pyrrolidinone
Catalog No.:BCN4155
CAS No.:62312-55-4
- Ursonic acid
Catalog No.:BCN4161
CAS No.:6246-46-4
- p-Vinylphenyl O-beta-D-glucopyranoside
Catalog No.:BCN1393
CAS No.:62470-46-6
- MK-0812
Catalog No.:BCC1755
CAS No.:624733-88-6
- 3-O-Acetyloleanderolide
Catalog No.:BCN4162
CAS No.:62498-83-3
- 23-O-Acetylshengmanol 3-O-beta-D-xyloside
Catalog No.:BCN7947
CAS No.:62498-88-8
- Gastrodin
Catalog No.:BCN6306
CAS No.:62499-27-8
- Parishin A
Catalog No.:BCN3811
CAS No.:62499-28-9
- Isodihydrofutoquinol B
Catalog No.:BCN6690
CAS No.:62499-71-2
- 4-Amino-4-methyl-2-pentanone
Catalog No.:BCN1772
CAS No.:625-04-7
- 3-Hydroxybutyric acid
Catalog No.:BCN2212
CAS No.:625-71-8
- Viniferol D
Catalog No.:BCN4164
CAS No.:625096-18-6
- Riociguat
Catalog No.:BCC1899
CAS No.:625115-55-1
Effect of delayed-release dimethyl fumarate on no evidence of disease activity in relapsing-remitting multiple sclerosis: integrated analysis of the phase III DEFINE and CONFIRM studies.[Pubmed:28328179]
Eur J Neurol. 2017 May;24(5):726-733.
BACKGROUND AND PURPOSE: Significant effects on clinical/neuroradiological disease activity have been reported in patients with relapsing-remitting multiple sclerosis treated with delayed-release Dimethyl Fumarate (DMF) in phase III DEFINE/CONFIRM trials. We conducted a post hoc analysis of integrated data from DEFINE/CONFIRM to evaluate the effect of DMF on achieving no evidence of disease activity (NEDA) in patients with relapsing-remitting multiple sclerosis. METHODS: The analysis included patients randomized to DMF 240 mg twice daily, placebo or glatiramer acetate (CONFIRM only) for
Comparative effectiveness of delayed-release dimethyl fumarate versus glatiramer acetate in multiple sclerosis patients: results of a matching-adjusted indirect comparison.[Pubmed:28350241]
J Comp Eff Res. 2017 Jun;6(4):313-323.
AIM: Using matching-adjusted indirect comparison, we compared efficacy outcomes in patients with relapsing-remitting multiple sclerosis treated with delayed-release Dimethyl Fumarate (DMF) or glatiramer acetate (GA). MATERIALS & METHODS: An indirect comparison of DMF (patient-level data) and GA (aggregate data) was conducted, with average baseline characteristics of DMF patients weighted to match those for GA patients. Direct comparison of DMF and GA was conducted in CONFIRM. Final results pooled the indirect and direct comparisons using meta-analysis. RESULTS: After matching, baseline characteristics were balanced between DMF and GA patients. Compared with GA, efficacy was significantly in favor of DMF as measured by annualized relapse rate (rate ratio: 0.76; 95% CI: 0.57-1.00; p = 0.0474) and 12-week confirmed disability progression (risk ratio: 0.59; 95% CI: 0.46-0.76; p < 0.0001). CONCLUSION: DMF demonstrated superior clinical efficacy versus GA.
Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway.[Pubmed:22267202]
J Pharmacol Exp Ther. 2012 Apr;341(1):274-84.
Oxidative stress is central to the pathology of several neurodegenerative diseases, including multiple sclerosis, and therapeutics designed to enhance antioxidant potential could have clinical value. The objective of this study was to characterize the potential direct neuroprotective effects of Dimethyl Fumarate (DMF) and its primary metabolite monomethyl fumarate (MMF) on cellular resistance to oxidative damage in primary cultures of central nervous system (CNS) cells and further explore the dependence and function of the nuclear factor (erythroid-derived 2)-like 2 (Nrf2) pathway in this process. Treatment of animals or primary cultures of CNS cells with DMF or MMF resulted in increased nuclear levels of active Nrf2, with subsequent up-regulation of canonical antioxidant target genes. DMF-dependent up-regulation of antioxidant genes in vivo was lost in mice lacking Nrf2 [Nrf2(-/-)]. DMF or MMF treatment increased cellular redox potential, glutathione, ATP levels, and mitochondrial membrane potential in a concentration-dependent manner. Treating astrocytes or neurons with DMF or MMF also significantly improved cell viability after toxic oxidative challenge in a concentration-dependent manner. This effect on viability was lost in cells that had eliminated or reduced Nrf2. These data suggest that DMF and MMF are cytoprotective for neurons and astrocytes against oxidative stress-induced cellular injury and loss, potentially via up-regulation of an Nrf2-dependent antioxidant response. These data also suggest DMF and MMF may function through improving mitochondrial function. The clinical utility of DMF in multiple sclerosis is being explored through phase III trials with BG-12, which is an oral therapeutic containing DMF as the active ingredient.
Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway.[Pubmed:21354971]
Brain. 2011 Mar;134(Pt 3):678-92.
Inflammation and oxidative stress are thought to promote tissue damage in multiple sclerosis. Thus, novel therapeutics enhancing cellular resistance to free radicals could prove useful for multiple sclerosis treatment. BG00012 is an oral formulation of dimethylfumarate. In a phase II multiple sclerosis trial, BG00012 demonstrated beneficial effects on relapse rate and magnetic resonance imaging markers indicative of inflammation as well as axonal destruction. First we have studied effects of dimethylfumarate on the disease course, central nervous system, tissue integrity and the molecular mechanism of action in an animal model of chronic multiple sclerosis: myelin oligodendrocyte glycoprotein induced experimental autoimmune encephalomyelitis in C57BL/6 mice. In the chronic phase of experimental autoimmune encephalomyelitis, preventive or therapeutic application of dimethylfumarate ameliorated the disease course and improved preservation of myelin, axons and neurons. In vitro, the application of fumarates increased murine neuronal survival and protected human or rodent astrocytes against oxidative stress. Application of dimethylfumarate led to stabilization of the transcription factor nuclear factor (erythroid-derived 2)-related factor 2, activation of nuclear factor (erythroid-derived 2)-related factor 2-dependent transcriptional activity and accumulation of NADP(H) quinoline oxidoreductase-1 as a prototypical target gene. Furthermore, the immediate metabolite of dimethylfumarate, monomethylfumarate, leads to direct modification of the inhibitor of nuclear factor (erythroid-derived 2)-related factor 2, Kelch-like ECH-associated protein 1, at cysteine residue 151. In turn, increased levels of nuclear factor (erythroid-derived 2)-related factor 2 and reduced protein nitrosylation were detected in the central nervous sytem of dimethylfumarate-treated mice. Nuclear factor (erythroid-derived 2)-related factor 2 was also upregulated in the spinal cord of autopsy specimens from untreated patients with multiple sclerosis. In dimethylfumarate-treated mice suffering from experimental autoimmune encephalomyelitis, increased immunoreactivity for nuclear factor (erythroid-derived 2)-related factor 2 was detected by confocal microscopy in neurons of the motor cortex and the brainstem as well as in oligodendrocytes and astrocytes. In mice deficient for nuclear factor (erythroid-derived 2)-related factor 2 on the same genetic background, the dimethylfumarate mediated beneficial effects on clinical course, axon preservation and astrocyte activation were almost completely abolished thus proving the functional relevance of this transcription factor for the neuroprotective mechanism of action. We conclude that the ability of dimethylfumarate to activate nuclear factor (erythroid-derived 2)-related factor 2 may offer a novel cytoprotective modality that further augments the natural antioxidant responses in multiple sclerosis tissue and is not yet targeted by other multiple sclerosis therapies.


