OxiracetamCAS# 62613-82-5 |
- Daptomycin
Catalog No.:BCC1057
CAS No.:103060-53-3
- Nelarabine
Catalog No.:BCC1072
CAS No.:121032-29-9
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- Ifosfamide
Catalog No.:BCC1164
CAS No.:3778-73-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 62613-82-5 | SDF | Download SDF |
| PubChem ID | 4626 | Appearance | Powder |
| Formula | C6H10N2O3 | M.Wt | 158.16 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 10 mg/mL (63.23 mM; Need ultrasonic and warming) | ||
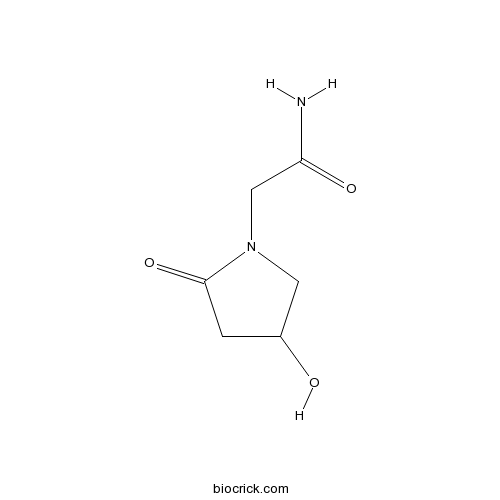
| Chemical Name | 2-(4-hydroxy-2-oxopyrrolidin-1-yl)acetamide | ||
| SMILES | C1C(CN(C1=O)CC(=O)N)O | ||
| Standard InChIKey | IHLAQQPQKRMGSS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C6H10N2O3/c7-5(10)3-8-2-4(9)1-6(8)11/h4,9H,1-3H2,(H2,7,10) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Oxiracetam is a cyclic derivative of gamma-aminobutyric acid (GABA). |

Oxiracetam Dilution Calculator

Oxiracetam Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.3227 mL | 31.6136 mL | 63.2271 mL | 126.4542 mL | 158.0678 mL |
| 5 mM | 1.2645 mL | 6.3227 mL | 12.6454 mL | 25.2908 mL | 31.6136 mL |
| 10 mM | 0.6323 mL | 3.1614 mL | 6.3227 mL | 12.6454 mL | 15.8068 mL |
| 50 mM | 0.1265 mL | 0.6323 mL | 1.2645 mL | 2.5291 mL | 3.1614 mL |
| 100 mM | 0.0632 mL | 0.3161 mL | 0.6323 mL | 1.2645 mL | 1.5807 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Oxiracetam, a cyclic derivative of gamma-aminobutyric acid (GABA), is used as a nootropic drug to improve memory and learning.
- H-HoCys-OH
Catalog No.:BCC3231
CAS No.:626-72-2
- 2,4-Dihydroxypyridine
Catalog No.:BCC8500
CAS No.:626-03-9
- Neocyclomorusin
Catalog No.:BCN3601
CAS No.:62596-35-4
- Cyclomorusin
Catalog No.:BCN4610
CAS No.:62596-34-3
- Morusin
Catalog No.:BCN4165
CAS No.:62596-29-6
- 11R,12-Dihydroxyspirovetiv-1(10)-en-2-one
Catalog No.:BCN1392
CAS No.:62574-30-5
- Captopril
Catalog No.:BCC2140
CAS No.:62571-86-2
- H-D-Phe(4-Br)-OH
Catalog No.:BCC3158
CAS No.:62561-74-4
- Isodihydrofutoquinol A
Catalog No.:BCN6691
CAS No.:62560-95-6
- Tariquidar methanesulfonate, hydrate
Catalog No.:BCC1986
CAS No.:625375-83-9
- Ethyl p-hydroxyphenyllactate
Catalog No.:BCN6654
CAS No.:62517-34-4
- Riociguat
Catalog No.:BCC1899
CAS No.:625115-55-1
- 2',4'-Dihydroxy-3'-methoxyacetophenone
Catalog No.:BCN7535
CAS No.:62615-26-3
- 4,6,7-Trimethoxy-5-methylcoumarin
Catalog No.:BCN4166
CAS No.:62615-63-8
- (+)-Mellein
Catalog No.:BCN7220
CAS No.:62623-84-1
- 11S,12-Dihydroxyspirovetiv-1(10)-en-2-one
Catalog No.:BCN1391
CAS No.:62623-86-3
- Ro 106-9920
Catalog No.:BCC7175
CAS No.:62645-28-7
- SIB 1893
Catalog No.:BCC6970
CAS No.:6266-99-5
- 1-Hydroxy-2-methylanthraquinone
Catalog No.:BCN3478
CAS No.:6268-09-3
- Handelin
Catalog No.:BCN2953
CAS No.:62687-22-3
- Saikosaponin F
Catalog No.:BCN2776
CAS No.:62687-63-2
- H-D-Arg-OH.HCl
Catalog No.:BCC2869
CAS No.:627-75-8
- Dioctanoylglycol
Catalog No.:BCC6662
CAS No.:627-86-1
- NF 449
Catalog No.:BCC7043
CAS No.:627034-85-9
Comparative pharmacokinetic studies of racemic oxiracetam and its pure enantiomers after oral administration in rats by a stereoselective HPLC method.[Pubmed:25886392]
J Pharm Biomed Anal. 2015;111:153-8.
Oxiracetam (ORC), a nootropic drug used for improving the cognition and memory, has an asymmetric carbon in its structure and exists as (S)- and (R)-ORC. The pharmacokinetic profiles of racemic Oxiracetam and its pure enantiomers in rats were evaluated and compared by enantioselective high-performance liquid chromatography, which was performed on a Chiralpak ID column with a mobile phase of hexane-ethanol-trifluoroacetic acid (78:22:0.1, v/v/v). The method was validated with respect to selectivity, linearity, accuracy and precision, stability and the limit of quantification. The validation acceptance criteria were met in all cases. A saturating phenomenon of (S)-ORC was observed when the dosage ranged from 200 mg/kg to 800 mg/kg. The two enantiomers showed similar profiles in the absorb phase, and reached the maximum concentration at 2h after oral administration. However, compared with the racemate group, the AUC/dose and Cmax/dose ratios of (S)-ORC were higher and Cl/f was lower in enanpure (S)-ORC group. The Cmax of (S)-ORC decreased from 21.3 +/- 5.0 mug/ml to 13.2 +/- 4.2 when (R)-ORC was co-administrated at the dose of 200mg/kg. AUC0-t values of (S)-ORC were different after oral administration of 200 mg/kg (S)-ORC and 400 mg/kg racemic ORC (96.7 +/- 15.5 and 50.1 +/- 16.3 mug h/ml). The higher absorption and slower elimination suggest that enantiopure (S)-ORC could be a promising drug that efficiently reduces clinical dosage, improves therapeutic indices, decreases toxicology risks, and results in increased therapeutic ration.
Enantioselective HPLC determination of oxiracetam enantiomers and application to a pharmacokinetic study in beagle dogs.[Pubmed:25978861]
J Chromatogr B Analyt Technol Biomed Life Sci. 2015 Jul 1;993-994:9-13.
An enantioselective high-performance liquid chromatography method was developed and validated for the determination of Oxiracetam enantiomers, a cognition and memory enhancer, in beagle dog plasma. The plasma samples were prepared by methanol extraction from 200muL plasma, and then the baseline resolution was achieved on a Chiralpak ID column (250mmx4.6mm, 5mum) with mobile phase of hexane-ethanol-trifluoroacetic acid (78:22:0.1, v/v/v) at flow rate of 1.0mL/min. The column elute was monitored using ultraviolet detection at 214nm. The method was linear over concentration range 0.50-100mug/mL for both enantiomers. The relative standard deviation values for intra- and inter-day precision were 0.78-13.61 and 0.74-8.92% for (R)- and (S)-Oxiracetam, respectively. The relative error values of accuracy ranged from -4.74 to 10.48% for (R)-Oxiracetam and from -0.19 to 11.48% for (S)-Oxiracetam. The method was successfully applied to a pharmacokinetic study of individual enantiomer and racemic Oxiracetam in beagle dogs after oral administration. The disposition of the two enantiomers was not stereoselective and chiral inversion was not observed in beagle dogs. The pharmacokinetic profiles of (S)-Oxiracetam were similar with racemic Oxiracetam in beagle dogs.
Pharmacokinetic comparisons of S-oxiracetam and R-oxiracetam in beagle dogs.[Pubmed:27279070]
Acta Pharm. 2016 Jun 1;66(2):279-87.
A pharmacokinetic comparison and conformational stability study of S-Oxiracetam (S-ORT) and R-Oxiracetam (R-ORT) in beagle dogs was used to investigate the possible mechanism of different effects of two Oxiracetam enantiomers through a random crossover design. After drug administration to beagle dogs, blood samples were collected at different time points for pharmacokinetic analysis using the UPLC-ESI-MS/MS method. Parts of plasma samples were used for conformation transformation studies using a normal phase high performance liquid chromatographic (NP HPLC) method. The study showed that Oxiracetam enantiomers maintained their original conformation when administered orally to beagle dogs. Concentrations of S-ORT were significantly higher than R-ORT 1.5 and 2 h after administration; the AUC0-infinity of S-ORT after oral administration tended to be higher than that of R-ORT, which showed that the different effects between S-ORT and R-ORT may be partly associated with their distinctive absorption at least.
Oxiracetam can improve cognitive impairment after chronic cerebral hypoperfusion in rats.[Pubmed:27741481]
Psychiatry Res. 2016 Dec 30;246:284-292.
Chronic cerebral hypoperfusion (CCH) induces cognitive deficits. Although CCH can be improved, cognitive impairment is not improved accordingly. To date, many studies have focused on investigating the pathophysiological mechanisms of CCH; however, the treatment of the induced cognitive impairment remains ineffective. Thus, the mechanisms underlying cognitive impairment after CCH and potential agents for treating this impairment need to be explored further. Oxiracetam is a nootropic drug that improves clinical outcomes for some central nervous system (CNS) disorders. Whether it can improve cognitive impairment after CCH is unknown. In this study, we used behavioural methods, electrophysiology, biochemistry, histopathological staining and transmission electron microscope to investigate rat's cognitive impairment by CCH, and found that Oxiracetam could improve CCH-induced cognitive impairment and prevent deficits of neural plasticity, white matter lesions, and synaptic ultrastructure. These results suggest that Oxiracetam may be effective as a potential agent against CCH-induced cognitive impairment.


