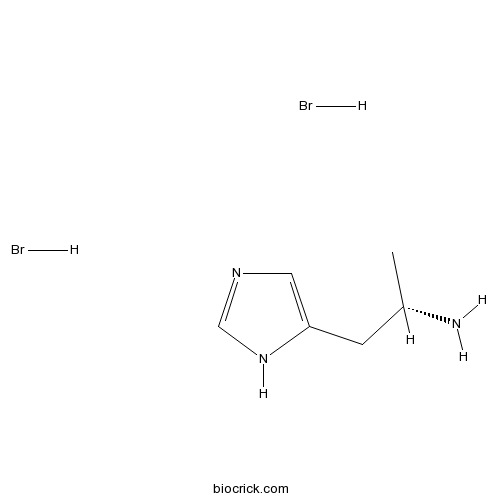
(S)-(+)-α-Methylhistamine dihydrobromideH3 agonist, less active enantiomer CAS# 75614-93-6 |
- I-BET-762
Catalog No.:BCC4474
CAS No.:1260907-17-2
- Bromodomain Inhibitor, (+)-JQ1
Catalog No.:BCC1132
CAS No.:1268524-70-4
- I-BET151 (GSK1210151A)
Catalog No.:BCC4476
CAS No.:1300031-49-5
- GSK1324726A
Catalog No.:BCC4038
CAS No.:1300031-52-0
- PFI-1 (PF-6405761)
Catalog No.:BCC2225
CAS No.:1403764-72-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 75614-93-6 | SDF | Download SDF |
| PubChem ID | 45073439 | Appearance | Powder |
| Formula | C6H13Br2N3 | M.Wt | 287 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
| Chemical Name | (2S)-1-(1H-imidazol-5-yl)propan-2-amine;dihydrobromide | ||
| SMILES | CC(CC1=CN=CN1)N.Br.Br | ||
| Standard InChIKey | RWHNAAABSGVRDT-XRIGFGBMSA-N | ||
| Standard InChI | InChI=1S/C6H11N3.2BrH/c1-5(7)2-6-3-8-4-9-6;;/h3-5H,2,7H2,1H3,(H,8,9);2*1H/t5-;;/m0../s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Less active enantiomer of H3 agonist R-(-)-α-methylhistamine; 120-fold less potent than R-(-) at H3. |

(S)-(+)-α-Methylhistamine dihydrobromide Dilution Calculator

(S)-(+)-α-Methylhistamine dihydrobromide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4843 mL | 17.4216 mL | 34.8432 mL | 69.6864 mL | 87.108 mL |
| 5 mM | 0.6969 mL | 3.4843 mL | 6.9686 mL | 13.9373 mL | 17.4216 mL |
| 10 mM | 0.3484 mL | 1.7422 mL | 3.4843 mL | 6.9686 mL | 8.7108 mL |
| 50 mM | 0.0697 mL | 0.3484 mL | 0.6969 mL | 1.3937 mL | 1.7422 mL |
| 100 mM | 0.0348 mL | 0.1742 mL | 0.3484 mL | 0.6969 mL | 0.8711 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Fludarabine Phosphate (Fludara)
Catalog No.:BCC3681
CAS No.:75607-67-9
- Methyl 3,4,5-trimethoxycinnamate
Catalog No.:BCN4589
CAS No.:7560-49-8
- Kaerophyllin
Catalog No.:BCN4304
CAS No.:75590-33-9
- alpha-Tocopherolquinone
Catalog No.:BCN4305
CAS No.:7559-04-8
- Sodium phosphate monobasic
Catalog No.:BCC8033
CAS No.:7558-80-7
- Sodium phosphate dibasic
Catalog No.:BCC7585
CAS No.:7558-79-4
- 20-Deoxyingenol 3-angelate
Catalog No.:BCN6642
CAS No.:75567-38-3
- Ingenol 3-Angelate
Catalog No.:BCN2961
CAS No.:75567-37-2
- Dencichin
Catalog No.:BCN2555
CAS No.:7554-90-7
- Moxonidine hydrochloride
Catalog No.:BCC5163
CAS No.:75536-04-8
- Nilvadipine
Catalog No.:BCC3799
CAS No.:75530-68-6
- Cedrin
Catalog No.:BCN4748
CAS No.:75513-81-4
- (-)-Usnic acid
Catalog No.:BCN4306
CAS No.:7562-61-0
- CHAPS
Catalog No.:BCC1476
CAS No.:75621-03-3
- Moracenin B
Catalog No.:BCC8341
CAS No.:75629-19-5
- Gomisin K1
Catalog No.:BCN7030
CAS No.:75629-20-8
- Oncrasin 1
Catalog No.:BCC2390
CAS No.:75629-57-1
- Knightolamine
Catalog No.:BCN1912
CAS No.:75638-70-9
- Chalcostrobamine
Catalog No.:BCN1900
CAS No.:75638-72-1
- DSLET
Catalog No.:BCC5718
CAS No.:75644-90-5
- Boc-D-Asn-OH
Catalog No.:BCC3362
CAS No.:75647-01-7
- Strobamine
Catalog No.:BCN1943
CAS No.:75656-91-6
- 4-Acetyl-1,1-dimethylpiperazinium iodide
Catalog No.:BCC6616
CAS No.:75667-84-4
- 2,4-Dihydroxy-4,6-dimethoxydihydrochalcone
Catalog No.:BCN1363
CAS No.:75679-58-2
Distribution, properties, and functional characteristics of three classes of histamine receptor.[Pubmed:2164693]
Pharmacol Rev. 1990 Mar;42(1):45-83.
It is clear from the preceding overview of histamine receptor pharmacology that research into the pharmacology of histamine receptors is at an exciting stage of development. The rapid advance of molecular biology should soon see the structural identification and cloning of all three of the major vertebrate histamine receptors. Further work will continue toward enhancing our understanding of the control by histamine of intracellular signaling via H1- and H2-receptors, and the rapid explosion of work on the H3-receptor should begin to unravel the mechanisms underlying its actions, perhaps via effects on ionic channels. The potential role of histamine as an intracellular second messenger raises exciting possibilities, as does the search for a histamine receptor analogous to the ligand-gated ion channel in the invertebrate nervous system.


