GYY 4137 morpholine saltSlow-releasing H2S donor CAS# 106740-09-4 |
- Pioglitazone HCl
Catalog No.:BCC2278
CAS No.:112529-15-4
- Rosiglitazone
Catalog No.:BCC2264
CAS No.:122320-73-4
- GW1929
Catalog No.:BCC1611
CAS No.:196808-24-9
- Inolitazone dihydrochloride
Catalog No.:BCC1653
CAS No.:223132-38-5
- T0070907
Catalog No.:BCC2261
CAS No.:313516-66-4
- Troglitazone
Catalog No.:BCC2016
CAS No.:97322-87-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 106740-09-4 | SDF | Download SDF |
| PubChem ID | 53393943 | Appearance | Powder |
| Formula | C15H25N2O3PS2 | M.Wt | 376.47 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
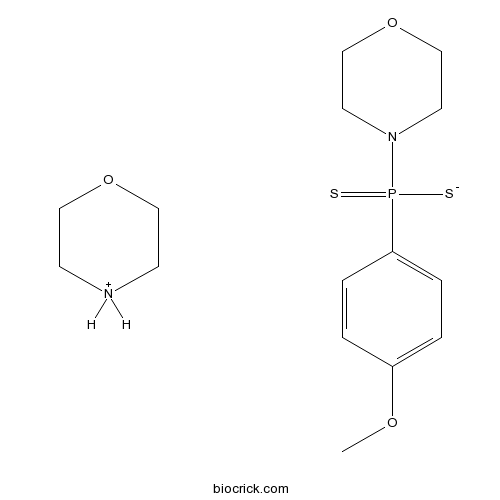
| Chemical Name | (4-methoxyphenyl)-morpholin-4-yl-sulfanylidene-sulfido-$l^{5}-phosphane;morpholin-4-ium | ||
| SMILES | COC1=CC=C(C=C1)P(=S)(N2CCOCC2)[S-].C1COCC[NH2+]1 | ||
| Standard InChIKey | YZMHNNLDUWRZFW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H16NO2PS2.C4H9NO/c1-13-10-2-4-11(5-3-10)15(16,17)12-6-8-14-9-7-12;1-3-6-4-2-5-1/h2-5H,6-9H2,1H3,(H,16,17);5H,1-4H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Slow-releasing H2S donor. Exhibits vasodilator and antihypertensive activity. Activity causes slow dilation of blood vessels in vitro and in vivo; does not influence vascular smooth muscle cell viability in culture. Water-soluble. |

GYY 4137 morpholine salt Dilution Calculator

GYY 4137 morpholine salt Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6563 mL | 13.2813 mL | 26.5625 mL | 53.1251 mL | 66.4064 mL |
| 5 mM | 0.5313 mL | 2.6563 mL | 5.3125 mL | 10.625 mL | 13.2813 mL |
| 10 mM | 0.2656 mL | 1.3281 mL | 2.6563 mL | 5.3125 mL | 6.6406 mL |
| 50 mM | 0.0531 mL | 0.2656 mL | 0.5313 mL | 1.0625 mL | 1.3281 mL |
| 100 mM | 0.0266 mL | 0.1328 mL | 0.2656 mL | 0.5313 mL | 0.6641 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Olprinone
Catalog No.:BCC1820
CAS No.:106730-54-5
- Boc-D-Lys-OH
Catalog No.:BCC3420
CAS No.:106719-44-2
- Matairesinol 4'-O-beta-gentiobioside
Catalog No.:BCN2848
CAS No.:106647-14-7
- Trachelosiaside
Catalog No.:BCN7743
CAS No.:106647-12-5
- Qingyangshengenin A
Catalog No.:BCN8126
CAS No.:106644-33-1
- 3,10-Dihydroxy-5,11-dielmenthadiene-4,9-dione
Catalog No.:BCN1633
CAS No.:106623-23-8
- Hederacolchiside A1
Catalog No.:BCN6553
CAS No.:106577-39-3
- Parstatin (mouse)
Catalog No.:BCC6042
CAS No.:1065756-01-5
- Parstatin (human)
Catalog No.:BCC6041
CAS No.:1065755-99-8
- 7-Amino-4-methylcoumarin-3-acetic acid
Catalog No.:BCN2562
CAS No.:106562-32-7
- SMND-309
Catalog No.:BCC1956
CAS No.:1065559-56-9
- Dafadine-A
Catalog No.:BCC5406
CAS No.:1065506-69-5
- 24-Deacetylalisol O
Catalog No.:BCN3365
CAS No.:1067510-31-9
- Otophylloside B
Catalog No.:BCN7267
CAS No.:106758-54-7
- Diethyl Acetamidomalonate
Catalog No.:BCC2841
CAS No.:1068-90-2
- BIBP 3226 trifluoroacetate
Catalog No.:BCC7456
CAS No.:1068148-47-9
- 5-Allyl-3-methoxy-6-methyl-7-(3,4,5-trimethoxyphenyl)bicyclo[3.2.1]oct-3-ene-2,8-dione
Catalog No.:BCN1632
CAS No.:106894-43-3
- Valproic acid sodium salt (Sodium valproate)
Catalog No.:BCC2156
CAS No.:1069-66-5
- Boc-D-Leucinol
Catalog No.:BCC2723
CAS No.:106930-51-2
- Adefovir
Catalog No.:BCC8808
CAS No.:106941-25-7
- Boc-isoleucinol
Catalog No.:BCC3096
CAS No.:106946-74-1
- Propylamine
Catalog No.:BCN1814
CAS No.:107-10-8
- Taurine
Catalog No.:BCN1750
CAS No.:107-35-7
- Betaine
Catalog No.:BCN6303
CAS No.:107-43-7
The hydrogen sulfide donor, GYY4137, exhibits anti-atherosclerotic activity in high fat fed apolipoprotein E(-/-) mice.[Pubmed:23713790]
Br J Pharmacol. 2013 Aug;169(8):1795-809.
BACKGROUND AND PURPOSE: Atherosclerosis is associated with reduced vascular hydrogen sulfide (H2 S) biosynthesis. GYY4137 is a novel slow-releasing H2 S compound that may effectively mimic the time course of H2 S release in vivo. However, it is not known whether GYY4137 affects atherosclerosis. EXPERIMENTAL APPROACH: RAW 264.7 cells and human blood monocyte-derived macrophages were incubated with oxidized low density lipoprotein (ox-LDL) with/without GYY4137. ApoE(-/-) mice were fed a high-fat diet for 4 weeks and administered GYY4137 for 30 days. Lipid and atherosclerotic lesions were measured by oil red O staining. Endothelium-dependent relaxation was assessed in response to acetylcholine. Superoxide production was detected by dihydroethidium staining. Expression of mRNA and protein were evaluated by quantitative real-time PCR and Western blot. KEY RESULTS: GYY4137 inhibited ox-LDL-induced foam cell formation and cholesterol esterification in cultured cells. GYY4137 decreased the expression of lectin-like ox-LDL receptor-1, iNOS, phosphorylated IkappaBalpha, NF-kappaB, ICAM-1, VCAM-1 and chemokines, including CXCL2, CXCR4, CXCL10 and CCL17, but increased the scavenger protein CD36, in ox-LDL-treated RAW 264.7 cells. In vivo, GYY4137 decreased aortic atherosclerotic plaque formation and partially restored aortic endothelium-dependent relaxation in apoE(-/-) mice. GYY4137 decreased ICAM-1, TNF-alpha and IL-6 mRNA expression as well as superoxide (O2 (-) ) generation in aorta. In addition, GYY4137 increased aortic eNOS phosphorylation and expression of PI3K, enhanced Akt Ser(473) phosphorylation and down-regulated the expression of LOX-1. CONCLUSION AND IMPLICATIONS: GYY4137 inhibits lipid accumulation induced by ox-LDL in RAW 264.7 cells. In vivo, GYY4137 decreased vascular inflammation and oxidative stress, improved endothelial function and reduced atherosclerotic plaque formation in apoE(-/-) mice.
The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo.[Pubmed:21701688]
PLoS One. 2011;6(6):e21077.
The slow-releasing hydrogen sulfide (H(2)S) donor, GYY4137, caused concentration-dependent killing of seven different human cancer cell lines (HeLa, HCT-116, Hep G2, HL-60, MCF-7, MV4-11 and U2OS) but did not affect survival of normal human lung fibroblasts (IMR90, WI-38) as determined by trypan blue exclusion. Sodium hydrosulfide (NaHS) was less potent and not active in all cell lines. A structural analogue of GYY4137 (ZYJ1122) lacking sulfur and thence not able to release H(2)S was inactive. Similar results were obtained using a clonogenic assay. Incubation of GYY4137 (400 microM) in culture medium led to the generation of low (<20 microM) concentrations of H(2)S sustained over 7 days. In contrast, incubation of NaHS (400 microM) in the same way led to much higher (up to 400 microM) concentrations of H(2)S which persisted for only 1 hour. Mechanistic studies revealed that GYY4137 (400 microM) incubated for 5 days with MCF-7 but not IMR90 cells caused the generation of cleaved PARP and cleaved caspase 9, indicative of a pro-apoptotic effect. GYY4137 (but not ZYJ1122) also caused partial G(2)/M arrest of these cells. Mice xenograft studies using HL-60 and MV4-11 cells showed that GYY4137 (100-300 mg/kg/day for 14 days) significantly reduced tumor growth. We conclude that GYY4137 exhibits anti-cancer activity by releasing H(2)S over a period of days. We also propose that a combination of apoptosis and cell cycle arrest contributes to this effect and that H(2)S donors should be investigated further as potential anti-cancer agents.
The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages.[Pubmed:19769459]
Antioxid Redox Signal. 2010 May 15;12(10):1147-54.
The role of hydrogen sulfide (H(2)S) in inflammation is controversial, with both pro- and antiinflammatory effects documented. Many studies have used simple sulfide salts as the source of H(2)S, which give a rapid bolus of H(2)S in aqueous solutions and thus do not accurately reflect the enzymatic generation of H(2)S. We therefore compared the effects of sodium hydrosulfide and a novel slow-releasing H(2)S donor (GYY4137) on the release of pro- and antiinflammatory mediators in lipopolysaccharide (LPS)-treated murine RAW264.7 macrophages. For the first time, we show that GYY4137 significantly and concentration-dependently inhibits LPS-induced release of proinflammatory mediators such as IL-1beta, IL-6, TNF-alpha, nitric oxide (*NO), and PGE(2) but increased the synthesis of the antiinflammatory chemokine IL-10 through NF-kappaB/ATF-2/HSP-27-dependent pathways. In contrast, NaHS elicited a biphasic effect on proinflammatory mediators and, at high concentrations, increased the synthesis of IL-1beta, IL-6, NO, PGE(2) and TNF-alpha. This study clearly shows that the effects of H(2)S on the inflammatory process are complex and dependent not only on H(2)S concentration but also on the rate of H(2)S generation. This study may also explain some of the apparent discrepancies in the literature regarding the pro- versus antiinflammatory role of H(2)S.
Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide.[Pubmed:18443240]
Circulation. 2008 May 6;117(18):2351-60.
BACKGROUND: The potential biological significance of hydrogen sulfide (H(2)S) has attracted growing interest in recent years. The aim of this study was to characterize a novel, water-soluble, slow-releasing H(2)S compound [morpholin-4-ium 4 methoxyphenyl(morpholino) phosphinodithioate (GYY4137)] and evaluate its use as a tool to investigate the cardiovascular biology of this gas. METHODS AND RESULTS: The acute vasorelaxant effect of drugs was assessed in rat aortic rings and perfused rat kidney in vitro and in the anesthetized rat in vivo. The chronic effect of GYY4137 on blood pressure in normotensive and spontaneously hypertensive rats was determined by tail-cuff plethysmography. GYY4137 released H(2)S slowly both in aqueous solution in vitro and after intravenous or intraperitoneal administration in anesthetized rats in vivo. GYY4137 caused a slow relaxation of rat aortic rings and dilated the perfused rat renal vasculature by opening vascular smooth muscle K(ATP) channels. GYY4137 did not affect rat heart rate or force of contraction in vitro. GYY4137 exhibited antihypertensive activity as evidenced by ability to reduce N(G)-nitro-L-arginine methyl ester-evoked hypertension in the anesthetized rat and after chronic (14-day) administration in spontaneously hypertensive rats. CONCLUSIONS: These results identify GYY4137 as a slow-releasing H(2)S compound with vasodilator and antihypertensive activity. GYY4137 is likely to prove useful in the study of the many and varied biological effects of H(2)S. GYY4137 may also prove of therapeutic value in cardiovascular disease.


