Procyanidin A1CAS# 103883-03-0 |
- Procyanidin A2
Catalog No.:BCN6805
CAS No.:41743-41-3
- Procyanidin A4
Catalog No.:BCN0293
CAS No.:111466-29-6
- Procyanidin A5
Catalog No.:BCN0892
CAS No.:111466-30-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

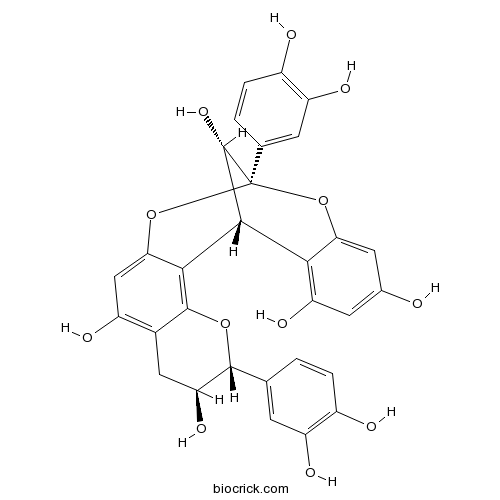
| Cas No. | 103883-03-0 | SDF | Download SDF |
| PubChem ID | 134715108 | Appearance | White-beige powder |
| Formula | C30H24O12 | M.Wt | 576.5 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | (+)-Epicatechin-(4β-8,2β-O-7)-catechin; Proanthocyanidin A1 | ||
| Solubility | Soluble in methanol; sparingly soluble in water | ||
| Chemical Name | (1R,5R,6S,13S,21S)-5,13-bis(3,4-dihydroxyphenyl)-4,12,14-trioxapentacyclo[11.7.1.02,11.03,8.015,20]henicosa-2(11),3(8),9,15,17,19-hexaene-6,9,17,19,21-pentol | ||
| SMILES | C1C(C(OC2=C3C4C(C(OC5=CC(=CC(=C45)O)O)(OC3=CC(=C21)O)C6=CC(=C(C=C6)O)O)O)C7=CC(=C(C=C7)O)O)O | ||
| Standard InChIKey | NSEWTSAADLNHNH-LQBIBLAPSA-N | ||
| Standard InChI | InChI=1S/C30H24O12/c31-13-7-20(37)24-22(8-13)41-30(12-2-4-16(33)19(36)6-12)29(39)26(24)25-23(42-30)10-17(34)14-9-21(38)27(40-28(14)25)11-1-3-15(32)18(35)5-11/h1-8,10,21,26-27,29,31-39H,9H2/t21-,26+,27+,29-,30-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Procyanidin A1 has antiallergic effects, it inhibits degranulation downstream of protein kinase C activation or Ca2⁺ influx from an internal store in RBL-2H3 cells. 2. Procyanidin A1 shows a very high inhibition effect on LDL oxidation, with the IC50 value of 0.94 uM. 3. Procyanidin A1, A2, B1 and B2 are potential precursors of 5-(3',4'-dihydroxyphenyl)-γ-valerolactone. |
| Targets | PKC | Calcium Channel | LDL | TNF-α | NF-kB | IkB | IKK |

Procyanidin A1 Dilution Calculator

Procyanidin A1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7346 mL | 8.673 mL | 17.3461 mL | 34.6921 mL | 43.3651 mL |
| 5 mM | 0.3469 mL | 1.7346 mL | 3.4692 mL | 6.9384 mL | 8.673 mL |
| 10 mM | 0.1735 mL | 0.8673 mL | 1.7346 mL | 3.4692 mL | 4.3365 mL |
| 50 mM | 0.0347 mL | 0.1735 mL | 0.3469 mL | 0.6938 mL | 0.8673 mL |
| 100 mM | 0.0173 mL | 0.0867 mL | 0.1735 mL | 0.3469 mL | 0.4337 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cycloart-25-ene-3,24-diol
Catalog No.:BCN5852
CAS No.:10388-48-4
- Lazabemide hydrochloride
Catalog No.:BCC7371
CAS No.:103878-83-7
- 4'-O-Methyllicoflavanone
Catalog No.:BCN4827
CAS No.:1038753-13-7
- 7-Prenylumbelliferone
Catalog No.:BCN2938
CAS No.:10387-50-5
- KX2-391 dihydrochloride
Catalog No.:BCC1686
CAS No.:1038395-65-1
- MBX-2982
Catalog No.:BCC1732
CAS No.:1037792-44-1
- Ganoderic acid C2
Catalog No.:BCN3036
CAS No.:103773-62-2
- Gimeracil
Catalog No.:BCC2296
CAS No.:103766-25-2
- R428
Catalog No.:BCC3692
CAS No.:1037624-75-1
- Shionone
Catalog No.:BCN1274
CAS No.:10376-48-4
- Fasudil
Catalog No.:BCC5262
CAS No.:103745-39-7
- Rehmaglutin D
Catalog No.:BCN5851
CAS No.:103744-84-9
- Lacidipine
Catalog No.:BCC4403
CAS No.:103890-78-4
- MK-4827
Catalog No.:BCC1761
CAS No.:1038915-60-4
- MK-4827 hydrochloride
Catalog No.:BCC4173
CAS No.:1038915-64-8
- MK-4827 tosylate
Catalog No.:BCC4174
CAS No.:1038915-73-9
- MK-4827 Racemate
Catalog No.:BCC5179
CAS No.:1038915-75-1
- Mannioside A
Catalog No.:BCN5853
CAS No.:1038922-95-0
- 17β-Hydroxy-17-methylandrosta-4,9(11)-dien-3-one
Catalog No.:BCC8444
CAS No.:1039-17-4
- Maxacalcitol
Catalog No.:BCC1730
CAS No.:103909-75-7
- (-)-Isodocarpin
Catalog No.:BCN3280
CAS No.:10391-08-9
- Nodosin
Catalog No.:BCN5854
CAS No.:10391-09-0
- Lupeol caffeate
Catalog No.:BCN5855
CAS No.:103917-26-6
- Oleanolic acid 3-O-beta-D-glucosyl-(1->3)-alpha-L-rhamnosyl(1->2)-alpha-L-arabinoside
Catalog No.:BCN8132
CAS No.:103956-33-8
Procyanidins from the stem wood of Machilus japonica and their inhibitory effect on LDL oxidation.[Pubmed:24297667]
Arch Pharm Res. 2014 Nov;37(11):1403-10.
The stem wood of Machilus japonica Siebold & Zucc were extracted with 80 % aqueous MeOH, and the concentrated extract was successively partitioned with ethyl acetate (EtOAc), normal butanol, and water. From the EtOAc fraction, five procyanidins, Procyanidin A1 (1), procyanidin A2 (2), procyanidin B7 (3), cinnamtannin B1 (4), and aesculitannin B (5), were isolated. Their chemical structures were identified through spectroscopic data analyses including NMR, MS, and IR. This is the first time any of these compounds have been isolated from this plant. The compounds were evaluated for inhibition activity on LDL oxidation. All of these compounds and the positive control, BHT, showed a very high inhibition effect with IC50 values of 0.94, 2.1, 1.8, 1.1, 1.0, and 1.9 muM, respectively.
Effects of peanut-skin procyanidin A1 on degranulation of RBL-2H3 cells.[Pubmed:21897038]
Biosci Biotechnol Biochem. 2011;75(9):1644-8.
Peanut skin contains large amounts of polyphenols having antiallergic effects. We found that a peanut-skin extract (PSE) inhibits the degranulation induced by antigen stimulation of rat basophilic leukemia (RBL-2H3) cells. A low-molecular-weight fraction from PSE, PSEL, also had inhibitory activity against allergic degranulation. A main polyphenol in PSEL was purified by gel chromatography and fractionated by YMC-gel ODS-AQ 120S50 column. Electrospray ionization mass spectrometry (ESI-MS) analysis of the purified polyphenol gave m/z 599 [M+Na](+). Based on the results of (1)H-NMR, (1)(3)C-NMR spectra, and optical rotation analysis, the polyphenol was identified as Procyanidin A1. It inhibited the degranulation caused by antigen stimulation at the IC(5)(0) of 20.3 microM. Phorbol-12-myristate-13-acetate (PMA) and 2,5,-di(tert-butyl)-1,4-hydroquinone (DTBHQ)-induced processes of degranulation were also inhibited by Procyanidin A1. These results indicate that peanut-skin Procyanidin A1 inhibits degranulation downstream of protein kinase C activation or Ca(2)(+) influx from an internal store in RBL-2H3 cells.
5-(3',4'-Dihydroxyphenyl-gamma-valerolactone), a Major Microbial Metabolite of Proanthocyanidin, Attenuates THP-1 Monocyte-Endothelial Adhesion.[Pubmed:28672844]
Int J Mol Sci. 2017 Jun 26;18(7). pii: ijms18071363.
Several metabolomics of polymeric flavan-3-ols have reported that proanthocyanidins are extensively metabolized by gut microbiota. 5-(3',4'-dihydroxyphenyl)-gamma-valerolactone (DHPV) has been reported to be the major microbial metabolite of proanthocyanidins. We demonstrated that DHPV has stronger prevention effect on tumor necrosis factor (TNF)-alpha-stimulated adhesion of THP-1 human monocytic cells to human umbilical vein endothelial cells compared to its potential precursors such as Procyanidin A1, A2, B1 and B2, (+)catechin, (-)epicatechin and its microbial metabolites such as 3-(3,4-dihydroxyphenyl)propionic acid and 2-(3,4-dihydroxyphenyl)acetic acid. Mechanism study showed that DHPV prevents THP-1 monocyte-endothelial cell adhesion by downregulating TNF-alpha-stimulated expressions of the two biomarkers of atherosclerosis such as vascular cell adhesion molecule-1 and monocyte chemotactic protein-1, activation of nuclear factor kappa B transcription and phosphorylation of I kappa-B kinase and IkappaBalpha. We suggested that DHPV has higher potentiality in prevention of atherosclerosis among the proanthocyanidin metabolites.


