Lazabemide hydrochlorideSelective MAO-B inhibitor CAS# 103878-83-7 |
- CUDC-101
Catalog No.:BCC2149
CAS No.:1012054-59-9
- Valproic acid sodium salt (Sodium valproate)
Catalog No.:BCC2156
CAS No.:1069-66-5
- M344
Catalog No.:BCC2162
CAS No.:251456-60-7
- Belinostat (PXD101)
Catalog No.:BCC2153
CAS No.:414864-00-9
- Trichostatin A (TSA)
Catalog No.:BCC3605
CAS No.:58880-19-6
- JNJ-26481585
Catalog No.:BCC2147
CAS No.:875320-29-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 103878-83-7 | SDF | Download SDF |
| PubChem ID | 163727 | Appearance | Powder |
| Formula | C8H11Cl2N3O | M.Wt | 236.1 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Ro 19-6327 | ||
| Solubility | Soluble to 100 mM in water and to 100 mM in DMSO | ||
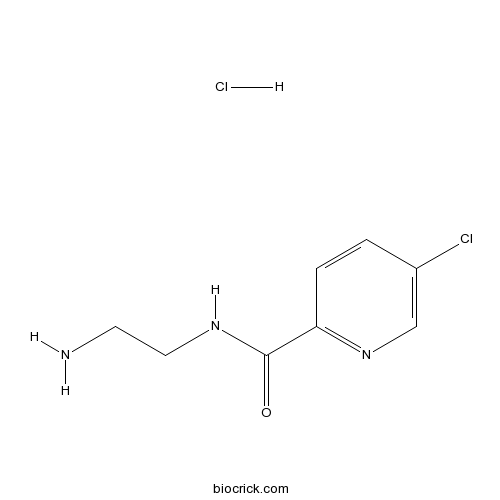
| Chemical Name | N-(2-aminoethyl)-5-chloropyridine-2-carboxamide;hydrochloride | ||
| SMILES | C1=CC(=NC=C1Cl)C(=O)NCCN.Cl | ||
| Standard InChIKey | JMFKTFLARGGXCC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H10ClN3O.ClH/c9-6-1-2-7(12-5-6)8(13)11-4-3-10;/h1-2,5H,3-4,10H2,(H,11,13);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective, reversible monoamine oxidase B (MAO-B) inhibitor (IC50 values are 0.03 and > 100 μM for MAO-B and MAO-A respectively). Inhibits monoamine uptake at high concentrations (IC50 values are 86, 123 and > 500 μM for noradrenalin, serotonin and dopamine uptake respectively). |

Lazabemide hydrochloride Dilution Calculator

Lazabemide hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.2355 mL | 21.1775 mL | 42.3549 mL | 84.7099 mL | 105.8873 mL |
| 5 mM | 0.8471 mL | 4.2355 mL | 8.471 mL | 16.942 mL | 21.1775 mL |
| 10 mM | 0.4235 mL | 2.1177 mL | 4.2355 mL | 8.471 mL | 10.5887 mL |
| 50 mM | 0.0847 mL | 0.4235 mL | 0.8471 mL | 1.6942 mL | 2.1177 mL |
| 100 mM | 0.0424 mL | 0.2118 mL | 0.4235 mL | 0.8471 mL | 1.0589 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4'-O-Methyllicoflavanone
Catalog No.:BCN4827
CAS No.:1038753-13-7
- 7-Prenylumbelliferone
Catalog No.:BCN2938
CAS No.:10387-50-5
- KX2-391 dihydrochloride
Catalog No.:BCC1686
CAS No.:1038395-65-1
- MBX-2982
Catalog No.:BCC1732
CAS No.:1037792-44-1
- Ganoderic acid C2
Catalog No.:BCN3036
CAS No.:103773-62-2
- Gimeracil
Catalog No.:BCC2296
CAS No.:103766-25-2
- R428
Catalog No.:BCC3692
CAS No.:1037624-75-1
- Shionone
Catalog No.:BCN1274
CAS No.:10376-48-4
- Fasudil
Catalog No.:BCC5262
CAS No.:103745-39-7
- Rehmaglutin D
Catalog No.:BCN5851
CAS No.:103744-84-9
- YK-4-279
Catalog No.:BCC2065
CAS No.:1037184-44-3
- Dihydrobonducellin
Catalog No.:BCN3731
CAS No.:103680-87-1
- Cycloart-25-ene-3,24-diol
Catalog No.:BCN5852
CAS No.:10388-48-4
- Procyanidin A1
Catalog No.:BCN6809
CAS No.:103883-03-0
- Lacidipine
Catalog No.:BCC4403
CAS No.:103890-78-4
- MK-4827
Catalog No.:BCC1761
CAS No.:1038915-60-4
- MK-4827 hydrochloride
Catalog No.:BCC4173
CAS No.:1038915-64-8
- MK-4827 tosylate
Catalog No.:BCC4174
CAS No.:1038915-73-9
- MK-4827 Racemate
Catalog No.:BCC5179
CAS No.:1038915-75-1
- Mannioside A
Catalog No.:BCN5853
CAS No.:1038922-95-0
- 17β-Hydroxy-17-methylandrosta-4,9(11)-dien-3-one
Catalog No.:BCC8444
CAS No.:1039-17-4
- Maxacalcitol
Catalog No.:BCC1730
CAS No.:103909-75-7
- (-)-Isodocarpin
Catalog No.:BCN3280
CAS No.:10391-08-9
- Nodosin
Catalog No.:BCN5854
CAS No.:10391-09-0
The activity of MAO A and B in rat renal cells and tubules.[Pubmed:9489509]
Life Sci. 1998;62(8):727-37.
The present study reports on the presence of type A and B monoamine oxidase (MAO) activity and their sensitivity to selective MAO-A and MAO-B inhibition by Ro 41-1049 and lazabemide, respectively, in homogenates of isolated rat renal tubules. Non-linear analysis of the saturation curve of H-5-hydroxytryptamine (3H-5-HT ) deamination revealed a Km of 351+/-71 microM (n=4) and a Vmax of 25+/-2 nmol mg protein(-1) h(-1). Deamination of 14C-beta-phenylethylamine (14C-beta-PEA) was also a saturable process yielding Km values of 58+/-12 microM and Vmax values of 24+/-2 nmol mg protein(-1) h(-1). Ro 41-1049 produced a concentration-dependent inhibition of 3H-5-HT deamination with a Ki of 24 nM. Deamination of 14C-beta-PEA was found to be reduced by lazabemide in a concentration-dependent manner with a Ki value of 17 nM. The effect of these selective MAO inhibitors on dopamine fate and DOPAC formation in isolated tubular epithelial cells was also studied. In these studies a clear inhibition of DOPAC formation was observed with Ro 41-1049 (250 nM), while 250 nM lazabemide was found not to increase the accumulation of newly-formed DA in those tubular epithelial cells loaded with 50 microM L-DOPA. In conclusion, the results presented here confirm the presence of both MAO-A and MAO-B activity in renal tubular epithelial cells, that MAO-A is the predominant enzyme involved in the deamination of the natriuretic hormone dopamine and that the deamination of newly-formed dopamine is a time-dependent process which occurs early after the decarboxylation of L-DOPA.
In vitro effects on monoamine uptake and release by the reversible monoamine oxidase-B inhibitors lazabemide and N-(2-aminoethyl)-p-chlorobenzamide: a comparison with L-deprenyl.[Pubmed:7605351]
Biochem Pharmacol. 1995 Jun 29;50(1):97-102.
To investigate whether the reversible monoamine oxidase-B (MAO-B) inhibitors lazabemide and Ro 16-6491 have any additional effect on monoamine uptake and release, in vitro experiments were performed on rat forebrain synaptosomes and blood platelets. The effects of the two drugs were compared with those of L-deprenyl, the well-known irreversible MAO-B inhibitor which is reported to affect amine uptake. Both lazabemide and Ro 16-6491 behaved as weak inhibitors of [3H]monoamine uptake by synaptosomes, with a similar rank order of potency for amine uptake inhibition (noradrenaline (NA) > or = 5-hydroxytryptamine (5 HT) > dopamine (DA)). The IC50 values for lazabemide and Ro 16-6491, respectively, were: 86 microM and 90 microM for NA uptake; 123 microM and 90 microM for 5HT uptake; > 500 microM and > 1000 microM for DA uptake. L-Deprenyl (rank order of inhibitory potency: NA > DA > 5 HT) was four to 10 times more potent than either compound in inhibiting [3H]catecholamine uptake (IC50 = NA 23 microM, DA 109 microM), and two to three times less potent in inhibiting 5 HT uptake (IC50 233 microM). Lazabemide and Ro 16-6491 also differed from L-deprenyl in their ability to induce release of endogenous monoamines from synaptosomes. Thus, Ro 16-6491 (500 microM) induced a greater 5 HT release than did L-deprenyl, but was less effective than L-deprenyl in releasing DA. On the contrary, lazabemide was almost completely inactive on either 5 HT and DA release. The differential effect of the three MAO-B inhibitors on synaptosome 5 HT uptake and release was confirmed by [14C]5HT uptake and liberation experiments with isolated rat platelets. The data indicate that the reversible MAO-B inhibitors lazabemide and Ro 16-6491 at relatively high concentrations possess amine uptake-inhibiting properties. With regard to the effects examined, lazabemide markedly differs from L-deprenyl since it does not interfere with DA uptake nor induce amine release from synaptosomes.
Quantitative enzyme radioautography with 3H-Ro 41-1049 and 3H-Ro 19-6327 in vitro: localization and abundance of MAO-A and MAO-B in rat CNS, peripheral organs, and human brain.[Pubmed:1578281]
J Neurosci. 1992 May;12(5):1977-99.
Monoamine oxidases A and B (MAO-A and MAO-B) oxidatively deaminate neurotransmitter and xenobiotic amines. Since the cellular localization of the isoenzymes in the CNS and peripheral organs determines to a large extent which substrate has access to which isoenzyme, knowledge of their tissue distribution and cellular localization is essential. Here we describe how reversible and selective inhibitors of MAO-A and MAO-B [Ro 41-1049 and Ro 19-6327 (lazabemide), respectively] can be used, as tritiated radioligands, to map the distribution and abundance of the enzymes in microscopic regions of the rat CNS and peripheral organs, and human brain by quantitative enzyme radioautography. The in vitro binding characteristics of both radiolabeled inhibitors revealed them to be selective, high-affinity ligands for the respective enzymes. KD and Bmax values for 3H-Ro 41-1049 in rat cerebral cortex were 10.7 nM and 7.38 pmol/mg protein, respectively, and for 3H-Ro 19-6327 were 18.4 nM and 3.45 pmol/mg protein, respectively. In accordance with their potencies as enzyme inhibitors, binding to MAO-A and MAO-B was competitively inhibited by clorgyline (IC50 = 1.4 nM) and L-deprenyl (selegiline; IC50 = 8.0 nM), respectively. The capacities of various rat and human tissues to bind the radioligands correlated extremely well with their corresponding enzyme activities. As revealed by the respective binding assays, the distribution and abundance of MAO-A and MAO-B in the tissues investigated differed markedly. MAO-A was most abundant in the locus coeruleus, paraventricular thalamus, bed nucleus of the stria terminalis, median habenular nucleus, ventromedial hypothalamus, raphe nuclei, solitary tract nucleus, inferior olives, interpeduncular nucleus, claustrum, and numerous peripheral tissues, including liver, vas deferens, heart, superior cervical ganglion, and exocrine and endocrine pancreas. In contrast, MAO-B was most abundant in the ependyma, circumventricular organs, olfactory nerve layer, periventricular hypothalamus, cingulum, hippocampal formation, raphe nuclei, paraventricular thalamus, mammillary nuclei, cerebellar Bergmann glia cells, liver, posterior pituitary, renal tubules, and endocrine pancreas. The cellular localization of the isoenzymes in both rat and human brain differs markedly and does not reflect the distribution of the presumed natural substrates, for example, absence of MAO-A in serotoninergic neurons. Indeed, the present evidence suggests that, whereas MAO-A is found in noradrenergic and adrenergic neurons, MAO-B occurs in astrocytes, serotoninergic neurons, as well as ventricular cells, including most circumventricular organs. The physiological roles of the enzymes are discussed in the light of these findings, some of which were unexpected.(ABSTRACT TRUNCATED AT 400 WORDS)


