Trazodone HClAntidepressant CAS# 25332-39-2 |
- Thrombin Receptor Agonist Peptide
Catalog No.:BCC3950
CAS No.:137339-65-2
- SLIGRL-NH2
Catalog No.:BCC3947
CAS No.:171436-38-7
- TFLLR-NH2
Catalog No.:BCC3948
CAS No.:197794-83-5
- AY-NH2
Catalog No.:BCC3949
CAS No.:352017-71-1
- ML161
Catalog No.:BCC3642
CAS No.:423735-93-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 25332-39-2 | SDF | Download SDF |
| PubChem ID | 62935 | Appearance | Powder |
| Formula | C19H23Cl2N5O | M.Wt | 408.32 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 16.67 mg/mL (40.83 mM; Need ultrasonic) H2O : 16.67 mg/mL (40.83 mM; Need ultrasonic) | ||
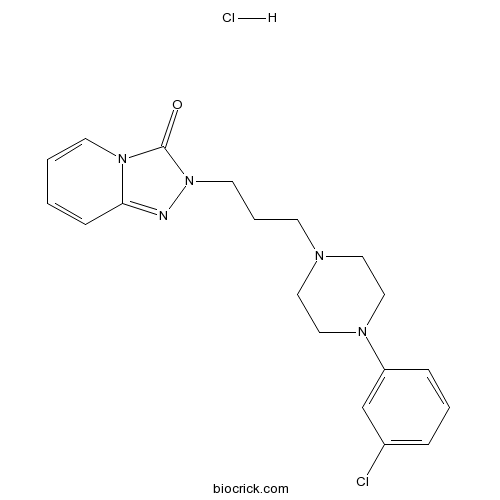
| Chemical Name | 2-[3-[4-(3-chlorophenyl)piperazin-1-yl]propyl]-[1,2,4]triazolo[4,3-a]pyridin-3-one;hydrochloride | ||
| SMILES | C1CN(CCN1CCCN2C(=O)N3C=CC=CC3=N2)C4=CC(=CC=C4)Cl.Cl | ||
| Standard InChIKey | OHHDIOKRWWOXMT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H22ClN5O.ClH/c20-16-5-3-6-17(15-16)23-13-11-22(12-14-23)8-4-10-25-19(26)24-9-2-1-7-18(24)21-25;/h1-3,5-7,9,15H,4,8,10-14H2;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 5-HT2A and α1 adrenoceptor antagonist. Enhances neuronal differentiation of mouse and human neural progenitor cells. Also inhibits PERK/eIF2α-P-mediated reduction in protein synthesis and restores memory deficits in a mouse dementia model. Antidepressant and neuroprotectant. |

Trazodone HCl Dilution Calculator

Trazodone HCl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4491 mL | 12.2453 mL | 24.4906 mL | 48.9812 mL | 61.2265 mL |
| 5 mM | 0.4898 mL | 2.4491 mL | 4.8981 mL | 9.7962 mL | 12.2453 mL |
| 10 mM | 0.2449 mL | 1.2245 mL | 2.4491 mL | 4.8981 mL | 6.1226 mL |
| 50 mM | 0.049 mL | 0.2449 mL | 0.4898 mL | 0.9796 mL | 1.2245 mL |
| 100 mM | 0.0245 mL | 0.1225 mL | 0.2449 mL | 0.4898 mL | 0.6123 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Trazodone HCl is an antidepressant belonging to the class of serotonin receptor antagonists and reuptake inhibitors for treatment of anxiety disorders.Trazodone is a triazolopyridine derivative, chemically and pharmacologically unrelated to other currentl
- BMS 195614
Catalog No.:BCC7740
CAS No.:253310-42-8
- Isocalamendiol
Catalog No.:BCN5119
CAS No.:25330-21-6
- Tetrabromobisphenol A diallyl ether
Catalog No.:BCC9174
CAS No.:25327-89-3
- Doxorubicin (Adriamycin) HCl
Catalog No.:BCC1117
CAS No.:25316-40-9
- Eribulin
Catalog No.:BCC5174
CAS No.:253128-41-5
- Luteosporin
Catalog No.:BCN5390
CAS No.:2530-39-4
- MRE 3008F20
Catalog No.:BCC6106
CAS No.:252979-43-4
- CHIR-98014
Catalog No.:BCC3751
CAS No.:252935-94-7
- CHIR-99021 (CT99021)
Catalog No.:BCC1275
CAS No.:252917-06-9
- TSU-68 (SU6668,Orantinib)
Catalog No.:BCC2508
CAS No.:252916-29-3
- RWJ 56110
Catalog No.:BCC7433
CAS No.:252889-88-6
- 20S,24R-Epoxydammar-12,25-diol-3-one
Catalog No.:BCN5118
CAS No.:25279-15-6
- Viniferin
Catalog No.:BCN3757
CAS No.:253435-07-3
- (+)-Corlumidine
Catalog No.:BCN2662
CAS No.:25344-54-1
- Wiskostatin
Catalog No.:BCC7934
CAS No.:253449-04-6
- 16-O-Acetylpolyporenic acid C
Catalog No.:BCN4058
CAS No.:2535-06-0
- 2-Amino-6-methylbenzothiazole
Catalog No.:BCC8543
CAS No.:2536-91-6
- Litseglutine B
Catalog No.:BCN5120
CAS No.:25368-01-8
- Asperulosidic acid
Catalog No.:BCN3088
CAS No.:25368-11-0
- TRIM
Catalog No.:BCC6847
CAS No.:25371-96-4
- AR-C 102222
Catalog No.:BCC6092
CAS No.:253771-21-0
- Kanamycin Sulfate
Catalog No.:BCC1205
CAS No.:25389-94-0
- Morroniside
Catalog No.:BCN5009
CAS No.:25406-64-8
- Boc-ß-HoAsp(OBzl)-OH
Catalog No.:BCC3229
CAS No.:254101-10-5
New colorimetric methods for the determination of trazodone HCl, famotidine, and diltiazem HCl in their pharmaceutical dosage forms.[Pubmed:12750873]
Anal Bioanal Chem. 2003 Jul;376(5):710-4.
Two sensitive and simple spectrophotometric methods are developed for the determination of Trazodone HCl, famotidine, and diltiazem HCl in pure and pharmaceutical preparations. The methods are based on the oxidation of the cited drugs with iron(III) in acidic medium. The liberated iron(II) reacts with 1,10-phenanthroline (method A) and the ferroin complex is colorimetrically measured at 510 nm against reagent blank. Method B is based on the reaction of the liberated Fe(II) with 2,2-bipyridyl to form a stable colored complex with lambda(max )at 520 nm. Optimization of the experimental conditions was described. Beer's law was obeyed in the concentration range of 1-5, 2-12, and 12-32 microg mL(-1) for trazodone, famotidine, and diltiazem with method A, and 1-10 and 8-16 microg mL(-1) for trazodone and famotidine with method B. The apparent molar absorptivity for method A is 1.06x10(5), 2.9x10(4), 1.2x10(4) and for method B is 9.4x10(4 )and 1.6x10(4), respectively. The suggested procedures could be used for the determination of trazodone, famotidine, and diltiazem, both in pure and dosage forms without interference from common excipients.
Voltammetric analysis of trazodone HCl in pharmaceuticals and biological fluids.[Pubmed:12191706]
J Pharm Biomed Anal. 2002 Sep 5;30(2):219-26.
The voltammetric behavior of trazodone (TRZ) HCl was studied using direct current (DC(t)), differential pulse (DPP) and alternating current (AC(t)) polarography. The drug manifests cathodic waves over the pH range of 10-14. The waves were characterized as being irreversible, diffusion-controlled with limited adsorption properties. At pH 10, the diffusion current-concentration relationship was found to be rectilinear over the range 4-32 and 0.8-24 microg ml(-1) using DC(t) and DPP modes, respectively, with minimum detectability (S/N=2) of 0.104 microg ml(-1) (2.45 x 10(-6) M) and 0.314 microg ml(-1) (7.397 x 10(-6) M) using the DPP and DC(t) modes, respectively. The diffusion-current constant (I(d)) is 4.31+/-0.02 (n=6). The proposed method was successfully applied to the determination of the studied compound either in pure form or in formulations. The results obtained were favorably compared with those given using a reference method. Furthermore, the proposed method was applied to the determination of TRZ in spiked human urine and plasma adopting the DPP technique. No prior extraction step is needed in case of urine. The percentage recoveries were 98.43+/-0.79 and 97.44+/-0.705 (n=4) in spiked human urine and plasma, respectively. A pathway for the electrode reaction was postulated.


