MRE 3008F20Highly potent and selective adenosine A3 receptor antagonist CAS# 252979-43-4 |
- MTEP hydrochloride
Catalog No.:BCC1780
CAS No.:1186195-60-7
- mGlu2 agonist
Catalog No.:BCC1745
CAS No.:1311385-32-6
- LY341495
Catalog No.:BCC1724
CAS No.:201943-63-7
- CPPHA
Catalog No.:BCC1501
CAS No.:693288-97-0
- Dipraglurant
Catalog No.:BCC1531
CAS No.:872363-17-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 252979-43-4 | SDF | Download SDF |
| PubChem ID | 5310960 | Appearance | Powder |
| Formula | C21H20N8O3 | M.Wt | 432.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in DMSO | ||
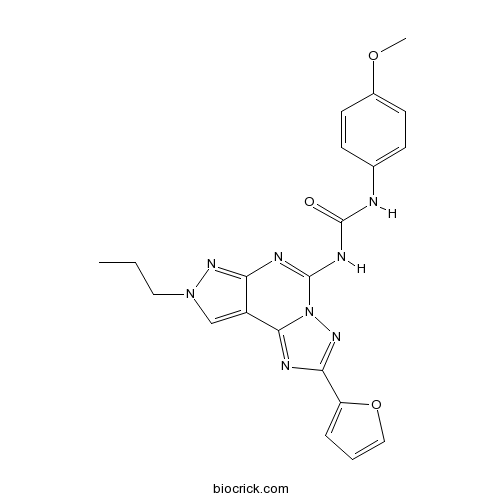
| SMILES | CCCN1C=C2C(=N1)N=C(N3C2=NC(=N3)C4=CC=CO4)NC(=O)NC5=CC=C(C=C5)OC | ||
| Standard InChIKey | CJRNHKSLHHWUAB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H20N8O3/c1-3-10-28-12-15-17(26-28)24-20(25-21(30)22-13-6-8-14(31-2)9-7-13)29-19(15)23-18(27-29)16-5-4-11-32-16/h4-9,11-12H,3,10H2,1-2H3,(H2,22,24,25,26,30) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent adenosine A3 receptor competitive antagonist. Selective for human A3 receptors over human A1 and A2A receptors (Ki values are 0.29, 141 and 1197 nM respectively). Potently inhibits agonist-induced cyclic AMP elevation in resting T lymphocytes (IC50 = 5 nM). |

MRE 3008F20 Dilution Calculator

MRE 3008F20 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3127 mL | 11.5634 mL | 23.1267 mL | 46.2535 mL | 57.8168 mL |
| 5 mM | 0.4625 mL | 2.3127 mL | 4.6253 mL | 9.2507 mL | 11.5634 mL |
| 10 mM | 0.2313 mL | 1.1563 mL | 2.3127 mL | 4.6253 mL | 5.7817 mL |
| 50 mM | 0.0463 mL | 0.2313 mL | 0.4625 mL | 0.9251 mL | 1.1563 mL |
| 100 mM | 0.0231 mL | 0.1156 mL | 0.2313 mL | 0.4625 mL | 0.5782 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- CHIR-98014
Catalog No.:BCC3751
CAS No.:252935-94-7
- CHIR-99021 (CT99021)
Catalog No.:BCC1275
CAS No.:252917-06-9
- TSU-68 (SU6668,Orantinib)
Catalog No.:BCC2508
CAS No.:252916-29-3
- RWJ 56110
Catalog No.:BCC7433
CAS No.:252889-88-6
- 20S,24R-Epoxydammar-12,25-diol-3-one
Catalog No.:BCN5118
CAS No.:25279-15-6
- Aristolene
Catalog No.:BCN8417
CAS No.:6831-16-9
- Apelin-36 (human)
Catalog No.:BCC5910
CAS No.:252642-12-9
- Ethyl orsellinate
Catalog No.:BCN4662
CAS No.:2524-37-0
- Lucidal
Catalog No.:BCN3206
CAS No.:252351-96-5
- Lucidadiol
Catalog No.:BCN7142
CAS No.:252351-95-4
- Isotaxiresinol 9,9'-acetonide
Catalog No.:BCN4663
CAS No.:252333-72-5
- 9,9'-O-Isopropyllidene-isolariciresinol
Catalog No.:BCN1474
CAS No.:252333-71-4
- Luteosporin
Catalog No.:BCN5390
CAS No.:2530-39-4
- Eribulin
Catalog No.:BCC5174
CAS No.:253128-41-5
- Doxorubicin (Adriamycin) HCl
Catalog No.:BCC1117
CAS No.:25316-40-9
- Tetrabromobisphenol A diallyl ether
Catalog No.:BCC9174
CAS No.:25327-89-3
- Isocalamendiol
Catalog No.:BCN5119
CAS No.:25330-21-6
- BMS 195614
Catalog No.:BCC7740
CAS No.:253310-42-8
- Trazodone HCl
Catalog No.:BCC5032
CAS No.:25332-39-2
- Viniferin
Catalog No.:BCN3757
CAS No.:253435-07-3
- (+)-Corlumidine
Catalog No.:BCN2662
CAS No.:25344-54-1
- Wiskostatin
Catalog No.:BCC7934
CAS No.:253449-04-6
- 16-O-Acetylpolyporenic acid C
Catalog No.:BCN4058
CAS No.:2535-06-0
- 2-Amino-6-methylbenzothiazole
Catalog No.:BCC8543
CAS No.:2536-91-6
[(3)H]MRE 3008F20: a novel antagonist radioligand for the pharmacological and biochemical characterization of human A(3) adenosine receptors.[Pubmed:10779381]
Mol Pharmacol. 2000 May;57(5):968-75.
The lack of a radiolabeled selective A(3) adenosine receptor antagonist is a major drawback for an adequate characterization of this receptor subtype. This paper describes the pharmacological and biochemical characterization of the tritiated form of a new potent A(3) adenosine receptor antagonist, the pyrazolo triazolo pyrimidine derivative [(3)H]5N-(4-methoxyphenylcarbamoyl)amino-8-propyl-2-(2-furyl )pyrazolo [4,3-e] -1,2,4- triazolo[1,5-c]pyrimidine ([(3)H]MRE 3008F20). [(3)H]MRE 3008F20 bound specifically to the human adenosine A(3) receptor expressed in CHO cells (hA(3)CHO), and saturation analysis revealed a single high affinity binding site, K(D) = 0.80 +/- 0.06 nM, with a B(max) = 300 +/- 33 fmol/mg protein. This new ligand displayed high selectivity (1294-, 165-, and 2471-fold) in binding assay to human A(3) versus A(1), A(2A), and A(2B) receptors, respectively, and binds to the rat A(3) receptors with a K(i) > 10 microM. The pharmacological profile of [(3)H]MRE 3008F20 binding to hA(3)CHO cells was evaluated using known adenosine receptor agonists and antagonists with a rank order of potency consistent with that typically found for interactions with the A(3) adenosine receptors. In the adenylyl cyclase assay the same compounds exhibited a rank order of potency identical with that observed in binding experiments. Thermodynamic data indicated that [(3)H]MRE 3008F20 binding to hA(3)CHO is entropy- and enthalpy-driven in agreement with the typical behavior of other adenosine antagonists to A(1) and A(2A) receptors. These results show that [(3)H]MRE 3008F20 is the first antagonist radioligand with high affinity and selectivity for the human A(3) adenosine receptor and may be used to investigate the physiopathological role of A(3) adenosine receptors.
Expression and functional role of adenosine receptors in regulating inflammatory responses in human synoviocytes.[Pubmed:20331607]
Br J Pharmacol. 2010 May;160(1):101-15.
BACKGROUND AND PURPOSE: Adenosine is an endogenous modulator, interacting with four G-protein coupled receptors (A(1), A(2A), A(2B) and A(3)) and acts as a potent inhibitor of inflammatory processes in several tissues. So far, the functional effects modulated by adenosine receptors on human synoviocytes have not been investigated in detail. We evaluated mRNA, the protein levels, the functional role of adenosine receptors and their pharmacological modulation in human synoviocytes. EXPERIMENTAL APPROACH: mRNA, Western blotting, saturation and competition binding experiments, cyclic AMP, p38 mitogen-activated protein kinases (MAPKs) and nuclear factor (NF)-kappaB activation, tumour necrosis factor alpha (TNF-alpha) and interleukin-8 (IL-8) release were assessed in human synoviocytes isolated from patients with osteoarthritis. KEY RESULTS: mRNA and protein for A(1), A(2A), A(2B) and A(3) adenosine receptors are expressed in human synoviocytes. Standard adenosine agonists and antagonists showed affinity values in the nanomolar range and were coupled to stimulation or inhibition of adenylyl cyclase. Activation of A(2A) and A(3) adenosine receptors inhibited p38 MAPK and NF-kappaB pathways, an effect abolished by selective adenosine antagonists. A(2A) and A(3) receptor agonists decreased TNF-alpha and IL-8 production. The phosphoinositide 3-kinase or G(s) pathways were involved in the functional responses of A(3) or A(2A) adenosine receptors. Synoviocyte A(1) and A(2B) adenosine receptors were not implicated in the inflammatory process whereas stimulation of A(2A) and A(3) adenosine receptors was closely associated with a down-regulation of the inflammatory status. CONCLUSIONS AND IMPLICATIONS: These results indicate that A(2A) and A(3) adenosine receptors may represent a potential target in therapeutic modulation of joint inflammation.
Expression of A3 adenosine receptors in human lymphocytes: up-regulation in T cell activation.[Pubmed:14978250]
Mol Pharmacol. 2004 Mar;65(3):711-9.
The present study investigates mRNA and protein levels of A3 adenosine receptors in resting (R) and activated (A) human lymphocytes. The receptors were evaluated by the antagonist radioligand [3H]5-N-(4-methoxyphenyl-carbamoyl)amino-8-propyl-2(2furyl)-pyrazolo-[4,3e]-1,2,4 -triazolo-[1,5-c]-pyrimidine ([3H]MRE 3008F20), which yielded Bmax values of 125 +/- 15 and 225 +/- 23 fmol/mg of protein and KD values of 1.79 +/- 0.30 and 1.85 +/- 0.25 nM in R and A cells, respectively. The protein seems to be induced with remarkable rapidity starting at 15 min and reaches a plateau at 30 min. Western blot assays revealed that the up-regulation of the A3 subtype after lymphocyte activation was caused by an increase in an enriched CD4+ cell fraction. Real-time reverse transcription-polymerase chain reaction experiments confirmed the rapid increase of A3 mRNA after T cell activation. Competition of radioligand binding by adenosine ligands displayed a rank order of potency typical of the A3 subtype. Thermodynamic data indicated that the binding is enthalpy- and entropy-driven in both R and A cells, suggesting that the activation process does not involve, at a molecular level, receptor alterations leading to modifications in the A3-related binding mechanisms. Functionally, the up-regulation of A3 adenosine receptors in A versus R cells corresponded to a potency increase of the A3 agonist N6-(3-iodo-benzyl)-2-chloro-adenosine-5'-N-methyluronamide in inhibiting cAMP accumulation (IC50=1.5 +/- 0.4 and 2.7 +/- 0.3 nM, respectively); this effect was antagonized by MRE 3008F20 (IC50=5.0 +/- 0.3 nM). In conclusion, our results provide, for the first time, an in-depth investigation of A3 receptors in human lymphocytes and demonstrate that, under activating conditions, they are up-regulated and may contribute to the effects triggered by adenosine.


