Ethyl orsellinateCAS# 2524-37-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2524-37-0 | SDF | Download SDF |
| PubChem ID | 75653 | Appearance | Powder |
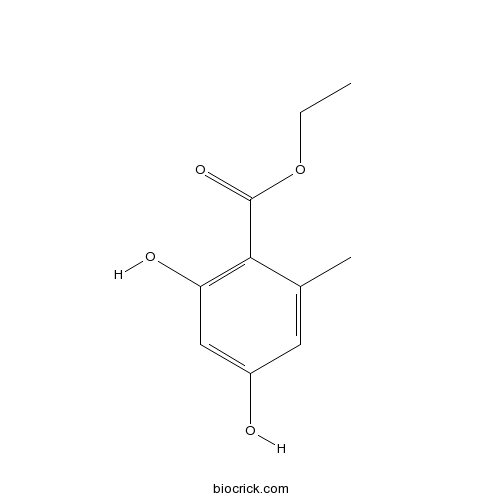
| Formula | C10H12O4 | M.Wt | 196.20 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | ethyl 2,4-dihydroxy-6-methylbenzoate | ||
| SMILES | CCOC(=O)C1=C(C=C(C=C1C)O)O | ||
| Standard InChIKey | UQSRXQMIXSZGLA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H12O4/c1-3-14-10(13)9-6(2)4-7(11)5-8(9)12/h4-5,11-12H,3H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ethyl orsellinate shows good inhibition of protein glycation, and urease activities. |
| In vitro | New antiglycation and enzyme inhibitors from Parmotrema cooperi[Reference: WebLink]Science China Chemistry, 2011 ,54 (12) :1926-1931.Lichens are unique individuals which have been widely used in traditional medicines. This study was focused on the bioassay-guided phytochemical investigation, and bioactivity evaluation on a lichens species, Parmotrema cooperi.
|
| Structure Identification | Zhongguo Zhong Yao Za Zhi. 2011 Aug;36(16):2233-5.Determination of atranol, lecanorin, ethyl orsellinate and methyl orsellinate in Usnea diffracta by RP-HPLC.[Pubmed: 22097337 ]To develop a RP-HPLC method for determining the contents of atranol, lecanorin, Ethyl orsellinate and mEthyl orsellinate in Usnea diffracta.
|

Ethyl orsellinate Dilution Calculator

Ethyl orsellinate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.0968 mL | 25.4842 mL | 50.9684 mL | 101.9368 mL | 127.421 mL |
| 5 mM | 1.0194 mL | 5.0968 mL | 10.1937 mL | 20.3874 mL | 25.4842 mL |
| 10 mM | 0.5097 mL | 2.5484 mL | 5.0968 mL | 10.1937 mL | 12.7421 mL |
| 50 mM | 0.1019 mL | 0.5097 mL | 1.0194 mL | 2.0387 mL | 2.5484 mL |
| 100 mM | 0.051 mL | 0.2548 mL | 0.5097 mL | 1.0194 mL | 1.2742 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Lucidal
Catalog No.:BCN3206
CAS No.:252351-96-5
- Lucidadiol
Catalog No.:BCN7142
CAS No.:252351-95-4
- Isotaxiresinol 9,9'-acetonide
Catalog No.:BCN4663
CAS No.:252333-72-5
- 9,9'-O-Isopropyllidene-isolariciresinol
Catalog No.:BCN1474
CAS No.:252333-71-4
- 1-Cinnamoylpyrrole
Catalog No.:BCN4006
CAS No.:252248-89-8
- Tertiapin-Q
Catalog No.:BCC5740
CAS No.:252198-49-5
- Fmoc-Lys(Me2)-OH
Catalog No.:BCC2567
CAS No.:252049-10-8
- Fmoc-D-Threoninol
Catalog No.:BCC2702
CAS No.:252049-02-8
- AZD7545
Catalog No.:BCC4294
CAS No.:252017-04-2
- A 205804
Catalog No.:BCC3944
CAS No.:251992-66-2
- SLV 320
Catalog No.:BCC7656
CAS No.:251945-92-3
- AM 1172
Catalog No.:BCC7675
CAS No.:251908-92-6
- Apelin-36 (human)
Catalog No.:BCC5910
CAS No.:252642-12-9
- Aristolene
Catalog No.:BCN8417
CAS No.:6831-16-9
- 20S,24R-Epoxydammar-12,25-diol-3-one
Catalog No.:BCN5118
CAS No.:25279-15-6
- RWJ 56110
Catalog No.:BCC7433
CAS No.:252889-88-6
- TSU-68 (SU6668,Orantinib)
Catalog No.:BCC2508
CAS No.:252916-29-3
- CHIR-99021 (CT99021)
Catalog No.:BCC1275
CAS No.:252917-06-9
- CHIR-98014
Catalog No.:BCC3751
CAS No.:252935-94-7
- MRE 3008F20
Catalog No.:BCC6106
CAS No.:252979-43-4
- Luteosporin
Catalog No.:BCN5390
CAS No.:2530-39-4
- Eribulin
Catalog No.:BCC5174
CAS No.:253128-41-5
- Doxorubicin (Adriamycin) HCl
Catalog No.:BCC1117
CAS No.:25316-40-9
- Tetrabromobisphenol A diallyl ether
Catalog No.:BCC9174
CAS No.:25327-89-3
[Determination of atranol, lecanorin, ethyl orsellinate and methyl orsellinate in Usnea diffracta by RP-HPLC].[Pubmed:22097337]
Zhongguo Zhong Yao Za Zhi. 2011 Aug;36(16):2233-5.
OBJECTIVE: To develop a RP-HPLC method for determining the contents of atranol, lecanorin, Ethyl orsellinate and mEthyl orsellinate in Usnea diffracta. METHOD: A Kromasil-C18 column (4.6 mm x 250 mm, 5 microm) was used at 25 degrees C with the mobile phases of acetonitrile -1% acetic acid in a gradient manner. The flow rate was set at 1.0 mL x min(-1). The detection wavelength was 280 nm. RESULT: The correlation coefficients of atranol, lecanorin, Ethyl orsellinate, and mEthyl orsellinate were higher than 0.999. Recoveries were from 102.9% to 95.30%; with RSD from 2.3% to 1.9%. CONCLUSION: The method is quick, simple and repeatable for simultaneous determination of atranol, lecanorin, Ethyl orsellinate and mEthyl orsellinate in U. diffracta.
[Study on the chemical constituents of two lichen plants from Meng Mountain].[Pubmed:24620693]
Zhong Yao Cai. 2013 Sep;36(9):1454-6.
OBJECTIVE: To investigate the chemical constituents of the lichen plants Parmelia tinctorum and Parmelia nimandairana collected from Meng Mountain in Shandong province. METHODS: Various chromatographic techniques were used to isolate and purify the constituents and their structures were elucidated by means of spectral evidence and physiochemical properties. RESULTS: Four compounds were isolated from Parmelia tinctorum and identified as: lecanoric acid (I), evernic acid (II), Ethyl orsellinate (III) and 3,5-dihydroxytoluene (IV). Two compounds were isolated from Parmelia nimandairana and identified as: usnic acid (V) and salazinic acid (VI). CONCLUSION: Compounds V and VI are isolated from Parmelia nimandairana for the first time.


