EB 47Potent PARP-1 inhibitor CAS# 1190332-25-2 |
- MK-4827
Catalog No.:BCC1761
CAS No.:1038915-60-4
- BMN-673 8R,9S
Catalog No.:BCC1422
CAS No.:1207456-00-5
- XAV-939
Catalog No.:BCC1120
CAS No.:284028-89-3
- PJ34
Catalog No.:BCC1865
CAS No.:344458-19-1
- ABT-888 (Veliparib)
Catalog No.:BCC1267
CAS No.:912444-00-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1190332-25-2 | SDF | Download SDF |
| PubChem ID | 124080891 | Appearance | Powder |
| Formula | C24H29Cl2N9O6 | M.Wt | 610.45 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 5 mM in water with gentle warming and to 50 mM in DMSO | ||
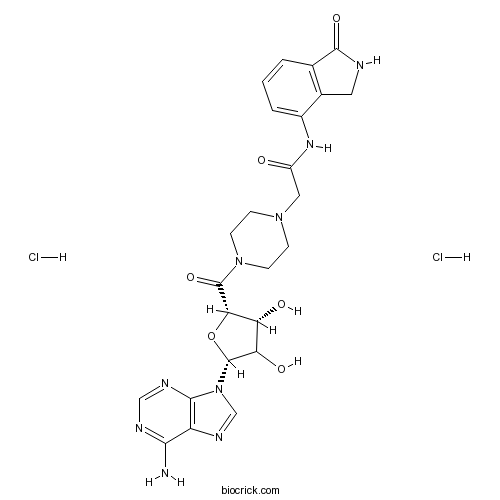
| Chemical Name | 2-[4-[(2S,3R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolane-2-carbonyl]piperazin-1-yl]-N-(1-oxo-2,3-dihydroisoindol-4-yl)acetamide;dihydrochloride | ||
| SMILES | C1CN(CCN1CC(=O)NC2=CC=CC3=C2CNC3=O)C(=O)C4C(C(C(O4)N5C=NC6=C5N=CN=C6N)O)O.Cl.Cl | ||
| Standard InChIKey | VVMQSDIMNDTMII-LLGQWWOSSA-N | ||
| Standard InChI | InChI=1S/C24H27N9O6.2ClH/c25-20-16-21(28-10-27-20)33(11-29-16)24-18(36)17(35)19(39-24)23(38)32-6-4-31(5-7-32)9-15(34)30-14-3-1-2-12-13(14)8-26-22(12)37;;/h1-3,10-11,17-19,24,35-36H,4-9H2,(H,26,37)(H,30,34)(H2,25,27,28);2*1H/t17-,18?,19+,24-;;/m1../s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent inhibitor of PARP-1 (IC50 = 45 nM). Reduces infarct volume in both a rat transient middle cerebral arterial occlusion model and a cardiac reperfusion model. |

EB 47 Dilution Calculator

EB 47 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6381 mL | 8.1907 mL | 16.3814 mL | 32.7627 mL | 40.9534 mL |
| 5 mM | 0.3276 mL | 1.6381 mL | 3.2763 mL | 6.5525 mL | 8.1907 mL |
| 10 mM | 0.1638 mL | 0.8191 mL | 1.6381 mL | 3.2763 mL | 4.0953 mL |
| 50 mM | 0.0328 mL | 0.1638 mL | 0.3276 mL | 0.6553 mL | 0.8191 mL |
| 100 mM | 0.0164 mL | 0.0819 mL | 0.1638 mL | 0.3276 mL | 0.4095 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
EB 47 is a potent inhibitor of PARP-1 with IC50 of 45 nM.
- PSI-7976
Catalog No.:BCC5138
CAS No.:1190308-01-0
- PSI-7977
Catalog No.:BCC1871
CAS No.:1190307-88-0
- Sarcandrone B
Catalog No.:BCN6074
CAS No.:1190225-48-9
- Sarcandrone A
Catalog No.:BCN6073
CAS No.:1190225-47-8
- M2 ion channel blocker
Catalog No.:BCC1726
CAS No.:1190215-03-2
- 4-[2-[(3-Ethyl-4-methyl-2-oxo-3-pyrrolin-1-yl)carboxamido]ethyl]benzenesulfonamide
Catalog No.:BCC8672
CAS No.:119018-29-0
- Abiesinol F
Catalog No.:BCN6418
CAS No.:1190070-91-7
- 2,2'-Biquinoline
Catalog No.:BCC8489
CAS No.:119-91-5
- 3,4-Dihydrocoumarin
Catalog No.:BCN6793
CAS No.:119-84-6
- 5-Amino-2-naphthalenesulfonic acid
Catalog No.:BCC8732
CAS No.:119-79-9
- 2-Carboxybenzaldehyde
Catalog No.:BCN2274
CAS No.:119-67-5
- Benzophenone
Catalog No.:BCC8859
CAS No.:119-61-9
- MRT67307
Catalog No.:BCC1779
CAS No.:1190378-57-4
- Euchrenone B1
Catalog No.:BCN3575
CAS No.:119061-09-5
- Fmoc-Asp-OH
Catalog No.:BCC3085
CAS No.:119062-05-4
- Fluorobexarotene
Catalog No.:BCC6110
CAS No.:1190848-23-7
- Phellolactone
Catalog No.:BCN3467
CAS No.:1190897-23-4
- Linolenic acid ethyl ester
Catalog No.:BCN8333
CAS No.:1191-41-9
- 1-Acetoxy-5-deacetylbaccatin I
Catalog No.:BCN6357
CAS No.:119120-27-3
- IOWH-032
Catalog No.:BCC3922
CAS No.:1191252-49-9
- Ganomycin I
Catalog No.:BCN3504
CAS No.:1191255-15-8
- B-Raf inhibitor 1 dihydrochloride
Catalog No.:BCC4183
CAS No.:1191385-19-9
- 3-Oxosapriparaquinone
Catalog No.:BCN3153
CAS No.:119139-56-9
- Glychionide A
Catalog No.:BCN3250
CAS No.:119152-50-0
Commentary on "African American men with very low-risk prostate cancer exhibit adverse oncologic outcomes after radical prostatectomy: should active surveillance still be an option for them?" Sundi D, Ross AE, Humphreys EB, Han M, Partin AW, Carter HB, Schaeffer EM, Johns Hopkins University, Baltimore, MD. J Clin Oncol 2013; 31(24):2991-7. [Epub 2013 Jun 17]. doi: 10.1200/JCO.2012.47.0302.[Pubmed:25087674]
Urol Oncol. 2014 Aug;32(6):936.
PURPOSE: Active surveillance (AS) is a treatment option for men with very low-risk prostate cancer (PCa); however, favorable outcomes achieved for men in AS are based on cohorts that under-represent African American (AA) men. To explore whether race-based health disparities exist among men with very low-risk PCa, we evaluated oncologic outcomes of AA men with very low-risk PCa who were candidates for AS but elected to undergo radical prostatectomy (RP). PATIENTS AND METHODS: We studied 1,801 men (256 AA, 1,473 white men, and 72 others) who met National Comprehensive Cancer Network criteria for very low-risk PCa and underwent RP. Presenting characteristics, pathologic data, and cancer recurrence were compared among the groups. Multivariable modeling was performed to assess the association of race with upgrading and adverse pathologic features. RESULTS: AA men with very low-risk PCa had more adverse pathologic features at RP and poorer oncologic outcomes. AA men were more likely to experience disease upgrading at prostatectomy (27.3% v 14.4%; P <.001), positive surgical margins (9.8% v 5.9%; P =.02), and higher Cancer of the Prostate Risk Assessment Post-Surgical scoring system (CAPRA-S) scores. On multivariable analysis, AA race was an independent predictor of adverse pathologic features (odds ratio, [OR], 3.23; P =.03) and pathologic upgrading (OR, 2.26; P =.03). CONCLUSION: AA men with very low-risk PCa who meet criteria for AS but undergo immediate surgery experience significantly higher rates of upgrading and adverse pathology than do white men and men of other races. AA men with very low-risk PCa should be counseled about increased oncologic risk when deciding among their disease management options.
Inhibitors of poly ADP-ribose polymerase (PARP) induce apoptosis of myeloid leukemic cells: potential for therapy of myeloid leukemia and myelodysplastic syndromes.[Pubmed:19407318]
Haematologica. 2009 May;94(5):638-46.
UNLABELLED: Background Aberrant or impaired repair of double-strand DNA breaks is a common feature of de novo acute myeloid leukemia and myelodysplastic syndromes. Since poly (ADP-ribose) polymerase (PARP) inhibitors have been recently shown to selectively target cells with defects in double-strand DNA repair, the aim of this study was to explore the possibility of exploiting defects in DNA repair in leukemic cells using PARP inhibitors. DESIGN AND METHODS: Leukemic cell lines were exposed to various PARP inhibitors alone and in combination with non-cytotoxic concentrations of DNA methyltransferase inhibitor, 5' aza-2'-deoxycytidine and/or the histone deacetylase inhibitor, MS275, to test for potentiation of apoptosis with these agents. RESULTS: PARP inhibitors, KU-0058948 and PJ34, induced cell cycle arrest and apoptosis of primary myeloid leukemic cells and myeloid leukemic cell lines in vitro. Immunofluorescence analysis also revealed that PARP inhibitor sensitivity in these leukemic cells was due to a defect in homologous recombination DNA repair. Addition of 5' aza-2'-deoxycytidine failed to increase the cytotoxicity of PARP inhibitors. In contrast, MS275 potentiated the cytotoxic effect of KU-0058948 and PJ34 in all PARP inhibitor-sensitive leukemic cells. Immunofluorescence analysis supported the idea that histone deacetylase inhibitors potentiate cytotoxicity by inhibiting DNA repair processes. Conclusions On the basis of the data presented here, we suggest that PARP inhibitors can potentially exploit defects in double-strand DNA break repair in leukemic cells, paving the way for testing the therapeutic potential of these agents in myelodysplastic syndromes and acute myeloid leukemia.
The discovery and synthesis of novel adenosine substituted 2,3-dihydro-1H-isoindol-1-ones: potent inhibitors of poly(ADP-ribose) polymerase-1 (PARP-1).[Pubmed:14684303]
Bioorg Med Chem Lett. 2004 Jan 5;14(1):81-5.
A series of novel 4-(N-acyl)-2,3-dihydro-1H-isoindol-1-ones have been prepared from methyl-3-nitro-2-methylbenzoate and linked through various spacers to the adenosine derivatives 11 and 12. We found that potent inhibition of poly(ADP-ribose)polymerase-1 (PARP-1) was achieved when isoindolinone was linked to adenosine by a spacer group of a specific length. Introduction of piperazine and succinyl linkers between the isoindolinone and adenosine core structures resulted in highly potent compounds 8a and 10b, which showed IC(50) values of 45 and 100 nM, respectively.


