MRT67307SIK/TBK-1/IKKe inhibitor CAS# 1190378-57-4 |
- Celastrol
Catalog No.:BCN5986
CAS No.:34157-83-0
- BMS-345541
Catalog No.:BCC1423
CAS No.:547757-23-3
- Bay 65-1942 free base
Catalog No.:BCC1408
CAS No.:600734-02-9
- Bay 65-1942 HCl salt
Catalog No.:BCC1409
CAS No.:600734-06-3
- Bay 65-1942 R form
Catalog No.:BCC1410
CAS No.:758683-21-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

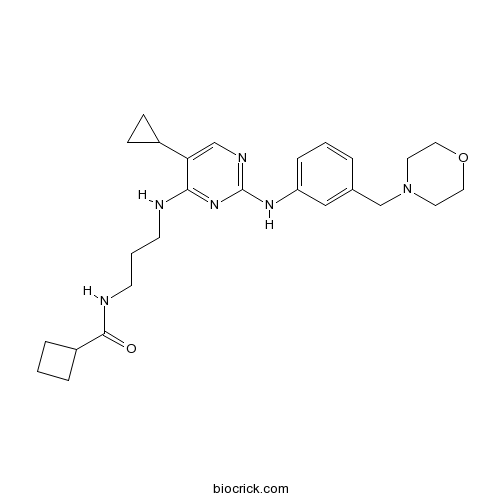
| Cas No. | 1190378-57-4 | SDF | Download SDF |
| PubChem ID | 44464263 | Appearance | Powder |
| Formula | C26H36N6O2 | M.Wt | 464.62 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (215.24 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | N-[3-[[5-cyclopropyl-2-[3-(morpholin-4-ylmethyl)anilino]pyrimidin-4-yl]amino]propyl]cyclobutanecarboxamide | ||
| SMILES | C1CC(C1)C(=O)NCCCNC2=NC(=NC=C2C3CC3)NC4=CC=CC(=C4)CN5CCOCC5 | ||
| Standard InChIKey | UKBGBACORPRCGG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C26H36N6O2/c33-25(21-5-2-6-21)28-11-3-10-27-24-23(20-8-9-20)17-29-26(31-24)30-22-7-1-4-19(16-22)18-32-12-14-34-15-13-32/h1,4,7,16-17,20-21H,2-3,5-6,8-15,18H2,(H,28,33)(H2,27,29,30,31) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | MRT67307 is a dual inhibitor of the IKKe and TBK-1 | |||||
| Targets | IKKe | TBK-1 | ||||

MRT67307 Dilution Calculator

MRT67307 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1523 mL | 10.7615 mL | 21.523 mL | 43.0459 mL | 53.8074 mL |
| 5 mM | 0.4305 mL | 2.1523 mL | 4.3046 mL | 8.6092 mL | 10.7615 mL |
| 10 mM | 0.2152 mL | 1.0761 mL | 2.1523 mL | 4.3046 mL | 5.3807 mL |
| 50 mM | 0.043 mL | 0.2152 mL | 0.4305 mL | 0.8609 mL | 1.0761 mL |
| 100 mM | 0.0215 mL | 0.1076 mL | 0.2152 mL | 0.4305 mL | 0.5381 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
MRT67307 is an inhibitor for TBK1, IKKε , MARK1-4 and NUAK1 with IC50 value of 19, 160, 27-52 and 230nM , respectively [1]. It is an inhibitor for ULK1and ULK2 with IC50 value of 45 and 38nM, respectively [2]. Also, it is a salt inducible kinase (SIK) inhibitor with IC50 value of 250, 67 and 430nM for SIK1, SIK2 and SIK3, respectively.
SIKs prevent the formation of regulatory macrophages and their inhibition induces increasing in some markers of regulatory macrophages, such as IL-10 and other anti-inflammatory molecules. IKKε and TBK-1 mediate the phosphorylation of interferon regulatory factor 3 (IRF3). MARK1 is a Serine/threonine-protein kinase.
In macrophages, MRT67307 prevented the production of IFNβ and the phosphorylation of IRF3 without suppressing the activation of NF-κB, which showed that MRT67307 blocked the induction of Pellino 1 through inhibiting TBK1/IKKε kinase activity [1] [3]. Also, MRT67307 completely blocked the TBK1- or IKKε-induced decrease in the mobility of Pellino 1 [3]. Exposed macrophages to MRT67307 increased the levels of the anti-inflammatory cytokines IL-1ra and IL-10 and decreased the levels of proinflammatory cytokines (such as IL-6, IL-12, and TNF) in response to bacterial lipopolysaccharide (LPS) [4].
References:
[1]. Clark K, Peggie M, Plater L, et al. Novel cross-talk within the IKK family controls innate immunity. Biochem J, 2011, 434(1): 93-104.
[2]. Petherick KJ, Conway OJ, Mpamhanga C, et al. Pharmacological Inhibition of ULK1 Blocks mTOR-Dependent Autophagy. J Biol Chem, 2015, pii: jbc.C114.627778.
[3]. Smith H, Liu XY, Dai L, et al. The role of TBK1 and IKKe in the expression and activation of Pellino 1. Biochem J, 2011, 434(3): 537-548.
[4]. Clark K, MacKenzie KF, Petkevicius K, et al. Phosphorylation of CRTC3 by the salt-inducible kinases controls the interconversion of classically activated and regulatory macrophages. Proc Natl Acad Sci U S A, 2012, 109(42): 16986-16991.
- EB 47
Catalog No.:BCC2452
CAS No.:1190332-25-2
- PSI-7976
Catalog No.:BCC5138
CAS No.:1190308-01-0
- PSI-7977
Catalog No.:BCC1871
CAS No.:1190307-88-0
- Sarcandrone B
Catalog No.:BCN6074
CAS No.:1190225-48-9
- Sarcandrone A
Catalog No.:BCN6073
CAS No.:1190225-47-8
- M2 ion channel blocker
Catalog No.:BCC1726
CAS No.:1190215-03-2
- 4-[2-[(3-Ethyl-4-methyl-2-oxo-3-pyrrolin-1-yl)carboxamido]ethyl]benzenesulfonamide
Catalog No.:BCC8672
CAS No.:119018-29-0
- Abiesinol F
Catalog No.:BCN6418
CAS No.:1190070-91-7
- 2,2'-Biquinoline
Catalog No.:BCC8489
CAS No.:119-91-5
- 3,4-Dihydrocoumarin
Catalog No.:BCN6793
CAS No.:119-84-6
- 5-Amino-2-naphthalenesulfonic acid
Catalog No.:BCC8732
CAS No.:119-79-9
- 2-Carboxybenzaldehyde
Catalog No.:BCN2274
CAS No.:119-67-5
- Euchrenone B1
Catalog No.:BCN3575
CAS No.:119061-09-5
- Fmoc-Asp-OH
Catalog No.:BCC3085
CAS No.:119062-05-4
- Fluorobexarotene
Catalog No.:BCC6110
CAS No.:1190848-23-7
- Phellolactone
Catalog No.:BCN3467
CAS No.:1190897-23-4
- Linolenic acid ethyl ester
Catalog No.:BCN8333
CAS No.:1191-41-9
- 1-Acetoxy-5-deacetylbaccatin I
Catalog No.:BCN6357
CAS No.:119120-27-3
- IOWH-032
Catalog No.:BCC3922
CAS No.:1191252-49-9
- Ganomycin I
Catalog No.:BCN3504
CAS No.:1191255-15-8
- B-Raf inhibitor 1 dihydrochloride
Catalog No.:BCC4183
CAS No.:1191385-19-9
- 3-Oxosapriparaquinone
Catalog No.:BCN3153
CAS No.:119139-56-9
- Glychionide A
Catalog No.:BCN3250
CAS No.:119152-50-0
- Coronarin A
Catalog No.:BCN6075
CAS No.:119188-33-9
Phosphorylation of CRTC3 by the salt-inducible kinases controls the interconversion of classically activated and regulatory macrophages.[Pubmed:23033494]
Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16986-91.
Macrophages acquire strikingly different properties that enable them to play key roles during the initiation, propagation, and resolution of inflammation. Classically activated (M1) macrophages produce proinflammatory mediators to combat invading pathogens and respond to tissue damage in the host, whereas regulatory macrophages (M2b) produce high levels of anti-inflammatory molecules, such as IL-10, and low levels of proinflammatory cytokines, like IL-12, and are important for the resolution of inflammatory responses. A central problem in this area is to understand how the formation of regulatory macrophages can be promoted at sites of inflammation to prevent and/or alleviate chronic inflammatory and autoimmune diseases. Here, we demonstrate that the salt-inducible kinases (SIKs) restrict the formation of regulatory macrophages and that their inhibition induces striking increases in many of the characteristic markers of regulatory macrophages, greatly stimulating the production of IL-10 and other anti-inflammatory molecules. We show that SIK inhibitors elevate IL-10 production by inducing the dephosphorylation of cAMP response element-binding protein (CREB)-regulated transcriptional coactivator (CRTC) 3, its dissociation from 14-3-3 proteins and its translocation to the nucleus where it enhances a gene transcription program controlled by CREB. Importantly, the effects of SIK inhibitors on IL-10 production are lost in macrophages that express a drug-resistant mutant of SIK2. These findings identify SIKs as a key molecular switch whose inhibition reprograms macrophages to an anti-inflammatory phenotype. The remarkable effects of SIK inhibitors on macrophage function suggest that drugs that target these protein kinases may have therapeutic potential for the treatment of inflammatory and autoimmune diseases.
Novel cross-talk within the IKK family controls innate immunity.[Pubmed:21138416]
Biochem J. 2011 Feb 15;434(1):93-104.
Members of the IKK {IkappaB [inhibitor of NF-kappaB (nuclear factor kappaB)] kinase} family play a central role in innate immunity by inducing NF-kappaB- and IRF [IFN (interferon) regulatory factor]-dependent gene transcription programmes required for the production of pro-inflammatory cytokines and IFNs. However, the molecular mechanisms that activate these protein kinases and their complement of physiological substrates remain poorly defined. Using MRT67307, a novel inhibitor of IKK/TBK1 (TANK {TRAF [TNF (tumour-necrosis-factor)-receptor-associated factor]-associated NF-kappaB activator}-binding kinase 1) and BI605906, a novel inhibitor of IKKbeta, we demonstrate that two different signalling pathways participate in the activation of the IKK-related protein kinases by ligands that activate the IL-1 (interleukin-1), TLR (Toll-like receptor) 3 and TLR4 receptors. One signalling pathway is mediated by the canonical IKKs, which directly phosphorylate and activate IKK and TBK1, whereas the second pathway appears to culminate in the autocatalytic activation of the IKK-related kinases. In contrast, the TNFalpha-induced activation of the IKK-related kinases is mediated solely by the canonical IKKs. In turn, the IKK-related kinases phosphorylate the catalytic subunits of the canonical IKKs and their regulatory subunit NEMO (NF-kappaB essential modulator), which is associated with reduced IKKalpha/beta activity and NF-kappaB-dependent gene transcription. We also show that the canonical IKKs and the IKK-related kinases not only have unique physiological substrates, such as IkappaBalpha, p105, RelA (IKKalpha and IKKbeta) and IRF3 (IKK and TBK1), but also have several substrates in common, including the catalytic and regulatory (NEMO and TANK) subunits of the IKKs themselves. Taken together, our studies reveal that the canonical IKKs and the IKK-related kinases regulate each other by an intricate network involving phosphorylation of their catalytic and regulatory (NEMO and TANK) subunits to balance their activities during innate immunity.
Pharmacological inhibition of ULK1 kinase blocks mammalian target of rapamycin (mTOR)-dependent autophagy.[Pubmed:25833948]
J Biol Chem. 2015 May 1;290(18):11376-83.
Autophagy is a cell-protective and degradative process that recycles damaged and long-lived cellular components. Cancer cells are thought to take advantage of autophagy to help them to cope with the stress of tumorigenesis; thus targeting autophagy is an attractive therapeutic approach. However, there are currently no specific inhibitors of autophagy. ULK1, a serine/threonine protein kinase, is essential for the initial stages of autophagy, and here we report that two compounds, MRT67307 and MRT68921, potently inhibit ULK1 and ULK2 in vitro and block autophagy in cells. Using a drug-resistant ULK1 mutant, we show that the autophagy-inhibiting capacity of the compounds is specifically through ULK1. ULK1 inhibition results in accumulation of stalled early autophagosomal structures, indicating a role for ULK1 in the maturation of autophagosomes as well as initiation.


