5-Amino-2-naphthalenesulfonic acidCAS# 119-79-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 119-79-9 | SDF | Download SDF |
| PubChem ID | 8408 | Appearance | Powder |
| Formula | C10H9NO3S | M.Wt | 223 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-aminonaphthalene-2-sulfonic acid | ||
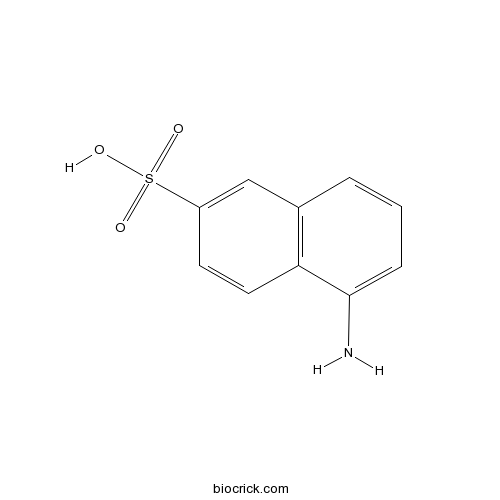
| SMILES | C1=CC2=C(C=CC(=C2)S(=O)(=O)O)C(=C1)N | ||
| Standard InChIKey | UWPJYQYRSWYIGZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H9NO3S/c11-10-3-1-2-7-6-8(15(12,13)14)4-5-9(7)10/h1-6H,11H2,(H,12,13,14) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

5-Amino-2-naphthalenesulfonic acid Dilution Calculator

5-Amino-2-naphthalenesulfonic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.4843 mL | 22.4215 mL | 44.843 mL | 89.6861 mL | 112.1076 mL |
| 5 mM | 0.8969 mL | 4.4843 mL | 8.9686 mL | 17.9372 mL | 22.4215 mL |
| 10 mM | 0.4484 mL | 2.2422 mL | 4.4843 mL | 8.9686 mL | 11.2108 mL |
| 50 mM | 0.0897 mL | 0.4484 mL | 0.8969 mL | 1.7937 mL | 2.2422 mL |
| 100 mM | 0.0448 mL | 0.2242 mL | 0.4484 mL | 0.8969 mL | 1.1211 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2-Carboxybenzaldehyde
Catalog No.:BCN2274
CAS No.:119-67-5
- Benzophenone
Catalog No.:BCC8859
CAS No.:119-61-9
- Benzoin
Catalog No.:BCC8854
CAS No.:119-53-9
- p-Anisoin
Catalog No.:BCC9113
CAS No.:119-52-8
- 7-Anilino-4-hydroxy-2-naphthalenesulfonic acid
Catalog No.:BCC8777
CAS No.:119-40-4
- Methyl salicylate
Catalog No.:BCN5372
CAS No.:119-36-8
- 8-Amino-2-naphthalenesulfonic acid
Catalog No.:BCC8783
CAS No.:119-28-8
- Viscumneoside III
Catalog No.:BCN7698
CAS No.:118985-27-6
- 1-O-Deacetyl-2alpha-hydroxykhayanolide E
Catalog No.:BCN1604
CAS No.:1189801-51-1
- Fumitremorgin C
Catalog No.:BCC7507
CAS No.:118974-02-0
- Mephedrone hydrochloride
Catalog No.:BCC6183
CAS No.:1189726-22-4
- Ethyl ganoderate J
Catalog No.:BCN3486
CAS No.:1189555-95-0
- 3,4-Dihydrocoumarin
Catalog No.:BCN6793
CAS No.:119-84-6
- 2,2'-Biquinoline
Catalog No.:BCC8489
CAS No.:119-91-5
- Abiesinol F
Catalog No.:BCN6418
CAS No.:1190070-91-7
- 4-[2-[(3-Ethyl-4-methyl-2-oxo-3-pyrrolin-1-yl)carboxamido]ethyl]benzenesulfonamide
Catalog No.:BCC8672
CAS No.:119018-29-0
- M2 ion channel blocker
Catalog No.:BCC1726
CAS No.:1190215-03-2
- Sarcandrone A
Catalog No.:BCN6073
CAS No.:1190225-47-8
- Sarcandrone B
Catalog No.:BCN6074
CAS No.:1190225-48-9
- PSI-7977
Catalog No.:BCC1871
CAS No.:1190307-88-0
- PSI-7976
Catalog No.:BCC5138
CAS No.:1190308-01-0
- EB 47
Catalog No.:BCC2452
CAS No.:1190332-25-2
- MRT67307
Catalog No.:BCC1779
CAS No.:1190378-57-4
- Euchrenone B1
Catalog No.:BCN3575
CAS No.:119061-09-5
Anti-angiogenic activity of heparin-like polysulfonated polymeric drugs in 3D human cell culture.[Pubmed:20674006]
Biomaterials. 2010 Nov;31(31):7863-72.
The activity of new anti-angiogenic polymeric drugs was tested in a 3D endothelial cell culture system applied as a model of angiogenesis. The assay was performed in a highly reproducible fibrin matrix that supported endothelial cell attachment, proliferation, migration, and formation of capillary-like structures. Active growth factors (FGF and/or VEGF) were added to the medium to induce the formation of blood vessel-like structures, and the effect of the active polymers was then tested by a semi-quantitative immunostaining protocol and visualized by laser-scanning confocal microscopy. The synthetic heparin-like macromolecules that were tested for their anti-angiogenic efficacy were previously characterized in terms of their anti-proliferative activity in 2D tissue culture. Two different anti-angiogenic monomers, a methacrylic derivative of 5-Amino-2-naphthalenesulfonic acid (MANSA) and 2-acrylamido-2-methylpropane sulfonic acid (AMPS), were copolymerized with a hydrophilic monomer (vinyl pyrrolidone, VP) or a hydrophobic monomer (butyl acrylate, BA), giving rise to different copolymeric systems with controlled microstructure and supramolecular organization. Both copolymeric systems have demonstrated a composition-dependent anti-mitogenic effect in "2D in vitro" cell culture experiments using aFGF as pro-angiogenic growth factor and BALB/c 3T3 fibroblast as cell model, as was shown in a previous publication. These 3D experiments provide evidence for the strong and specific modulation of angiogenesis by these systems. The 3D experiments constitute an improvement over 2D in vitro experiments and in vivo experiments with angiogenic drugs and may help to reduce the number of animal experiments.
An analytical system for the characterization of highly heterogeneous mixtures of N-linked oligosaccharides.[Pubmed:19281790]
Anal Biochem. 2009 Jun 1;389(1):40-51.
A novel system for characterizing complex N-linked oligosaccharide mixtures that uses a combination of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS), capillary electrophoresis (CE), and high-performance liquid chromatography (HPLC) has been developed. In this study, oligosaccharides released from recombinant TNK-tPA (tissue plasminogen activator) were derivatized with 5-Amino-2-naphthalenesulfonic acid (ANSA). The negative charge imparted by the ANSA label facilitated the analysis of the oligosaccharides by MALDI-TOF MS by allowing the observation of both neutral and sialylated oligosaccharides in a single negative ion mode spectrum. Labeling with ANSA was also determined to be advantageous in the characterization of oligosaccharides by both HPLC and CE. The ANSA label was demonstrated to provide superior resolution over the commonly used label 8-aminopyrene-1,3,6-trisulfonic acid (APTS) in both the CE and HPLC analysis of oligosaccharides. To date, no other labels that enable the analysis of complex oligosaccharide mixtures in a single mass spectral mode, while also enabling high-resolution chromatographic and electrophoretic separation of the oligosaccharides, have been reported. By integrating the structural information obtained by MALDI-TOF MS analysis with the ability of CE and HPLC to discriminate between structural isomers, the complete characterization of complex oligosaccharide mixtures is possible.
Poly(3,4-ethylenedioxythiophene-co-(5-amino-2-naphthalenesulfonic acid)) (PEDOT-PANS) film modified glassy carbon electrode for selective detection of dopamine in the presence of ascorbic acid and uric acid.[Pubmed:17616245]
Anal Chim Acta. 2007 Jul 16;596(1):92-8.
Poly(3,4-ethylenedioxythiophene-co-(5-Amino-2-naphthalenesulfonic acid)) (PEDOT-PANS) film modified glassy carbon electrode was prepared by electrochemical polymerization technique. The properties of modified electrode was studied. It was found that the electrochemical properties of modified electrode was very much dependent on the experimental conditions, such as monomer oxidation potential and pH. The modified electrode surface was characterized by scanning electron microscopy (SEM). The PEDOT-PANS film modified electrode shows electrocatalytic activity toward oxidation of dopamine (DA) in acetate buffer solution (pH 5.0) and results in a marked enhancement of the current response. The linear sweep voltammetric (LSV) peak heights are linear with DA concentration from 2x10(-6) to 1x10(-5) M. The detection limit is 5x10(-7) M. More over, the interferences of ascorbic acid (AA) and uric acid (UA) were effectively diminished. This work provides a simple and easy approach for selective determination of dopamine in the presence of ascorbic acid and uric acid.


