Methyl salicylateCAS# 119-36-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 119-36-8 | SDF | Download SDF |
| PubChem ID | 4133 | Appearance | Colorless liquid |
| Formula | C8H8O3 | M.Wt | 152.15 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Synonyms | Gaultheria Oil;68917-75-9;Methyl 2-Hydroxybenzoate; 119-36-8; Wintergreen Oil | ||
| Solubility | Ethanol : 25 mg/mL (164.31 mM; Need ultrasonic) | ||
| Chemical Name | methyl 2-hydroxybenzoate | ||
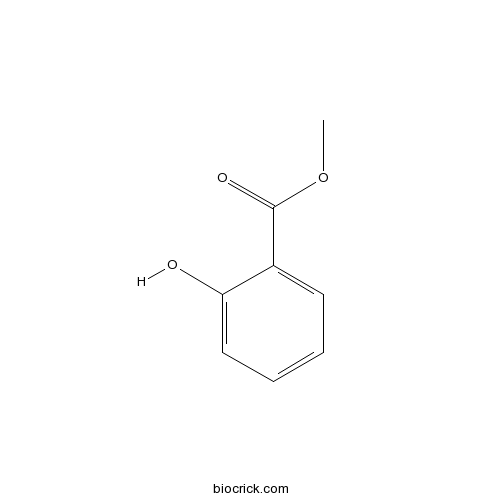
| SMILES | COC(=O)C1=CC=CC=C1O | ||
| Standard InChIKey | OSWPMRLSEDHDFF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H8O3/c1-11-8(10)6-4-2-3-5-7(6)9/h2-5,9H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Methyl salicylate is a common herbivore-induced plant volatile that, when applied to crops, has the potential to enhance natural enemy abundance and pest control. Methyl salicylate has both stimulatory and inhibitory actions on TRPV1 channels, it shows analgesic effects. |
| Targets | Estrogen receptor | NF-kB | TNF-α | Progestogen receptor |
| In vitro | Plant methyl salicylate induces defense responses in the rhizobacterium Bacillus subtilis.[Pubmed: 25181478]Environ Microbiol. 2015 Apr;17(4):1365-76.Bacillus subtilis is a rhizobacterium that promotes plant growth and health. Cultivation of B. subtilis with an uprooted weed on solid medium produced pleat-like architectures on colonies near the plant. To test whether plants emit signals that affect B. subtilis colony morphology, we examined the effect of plant-related compounds on colony morphology.
Hair analysis as a useful procedure for detection of vapour exposure to chemical warfare agents: simulation of sulphur mustard with methyl salicylate.[Pubmed: 24817050 ]Drug Test Anal. 2014 Jun;6 Suppl 1:67-73.Chemical warfare agents (CWA) are highly toxic compounds which have been produced to kill or hurt people during conflicts or terrorist attacks.
Despite the fact that their use is strictly prohibited according to international convention, populations' exposure still recently occurred. Development of markers of exposure to CWA is necessary to distinguish exposed victims from unexposed ones.
Fumigant Activity of 6 Selected Essential Oil Compounds and Combined Effect of Methyl Salicylate And Trans-Cinnamaldehyde Against Culex pipiens pallens.[Pubmed: 25843095]J Am Mosq Control Assoc. 2014 Sep;30(3):199-203.We studied the knockdown activity and lethal toxicity of 6 essential oil compounds-Methyl salicylate, linalool, 2-phenethyl alcohol, eugenol, β-citronellol, and trans-cinnamaldehyde-as fumigants against adult female Culex pipiens pallens in the laboratory.
|
| Cell Research | Involvement of transient receptor potential vanilloid subtype 1 in analgesic action of methyl salicylate.[Pubmed: 18987162 ]Mol Pharmacol. 2009 Feb;75(2):307-17.Methyl salicylate (MS) is a naturally occurring compound that is used as a major active ingredient of balms and liniments supplied as topical analgesics. Despite the common use of MS as a pain reliever, the underlying molecular mechanism is not fully understood.
Here we characterize the action of MS on transient receptor potential V1 (TRPV1).
|

Methyl salicylate Dilution Calculator

Methyl salicylate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.5725 mL | 32.8623 mL | 65.7246 mL | 131.4492 mL | 164.3115 mL |
| 5 mM | 1.3145 mL | 6.5725 mL | 13.1449 mL | 26.2898 mL | 32.8623 mL |
| 10 mM | 0.6572 mL | 3.2862 mL | 6.5725 mL | 13.1449 mL | 16.4312 mL |
| 50 mM | 0.1314 mL | 0.6572 mL | 1.3145 mL | 2.629 mL | 3.2862 mL |
| 100 mM | 0.0657 mL | 0.3286 mL | 0.6572 mL | 1.3145 mL | 1.6431 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 8-Amino-2-naphthalenesulfonic acid
Catalog No.:BCC8783
CAS No.:119-28-8
- Viscumneoside III
Catalog No.:BCN7698
CAS No.:118985-27-6
- 1-O-Deacetyl-2alpha-hydroxykhayanolide E
Catalog No.:BCN1604
CAS No.:1189801-51-1
- Fumitremorgin C
Catalog No.:BCC7507
CAS No.:118974-02-0
- Mephedrone hydrochloride
Catalog No.:BCC6183
CAS No.:1189726-22-4
- Ethyl ganoderate J
Catalog No.:BCN3486
CAS No.:1189555-95-0
- (S,S)-2,6-Bis(4-isopropyl-2-oxazolin-2-yl)pyridine
Catalog No.:BCC8402
CAS No.:118949-61-4
- 1,3-Dihydroxy-4-methoxy-10-methylacridin-9(10H)-one
Catalog No.:BCN1605
CAS No.:1189362-86-4
- 5-(3-Hydroxypropyl)-7-methoxybenzofuran
Catalog No.:BCN1606
CAS No.:118930-92-0
- Balanophonin
Catalog No.:BCN6072
CAS No.:118916-57-7
- Denudaquinol
Catalog No.:BCN8035
CAS No.:1189105-40-5
- Lettowienolide
Catalog No.:BCN8038
CAS No.:1189105-39-2
- 7-Anilino-4-hydroxy-2-naphthalenesulfonic acid
Catalog No.:BCC8777
CAS No.:119-40-4
- p-Anisoin
Catalog No.:BCC9113
CAS No.:119-52-8
- Benzoin
Catalog No.:BCC8854
CAS No.:119-53-9
- Benzophenone
Catalog No.:BCC8859
CAS No.:119-61-9
- 2-Carboxybenzaldehyde
Catalog No.:BCN2274
CAS No.:119-67-5
- 5-Amino-2-naphthalenesulfonic acid
Catalog No.:BCC8732
CAS No.:119-79-9
- 3,4-Dihydrocoumarin
Catalog No.:BCN6793
CAS No.:119-84-6
- 2,2'-Biquinoline
Catalog No.:BCC8489
CAS No.:119-91-5
- Abiesinol F
Catalog No.:BCN6418
CAS No.:1190070-91-7
- 4-[2-[(3-Ethyl-4-methyl-2-oxo-3-pyrrolin-1-yl)carboxamido]ethyl]benzenesulfonamide
Catalog No.:BCC8672
CAS No.:119018-29-0
- M2 ion channel blocker
Catalog No.:BCC1726
CAS No.:1190215-03-2
- Sarcandrone A
Catalog No.:BCN6073
CAS No.:1190225-47-8
Effect of methyl salicylate-based lures on beneficial and pest arthropods in strawberry.[Pubmed:20388299]
Environ Entomol. 2010 Apr;39(2):653-60.
Methyl salicylate (MeSA) is a common herbivore-induced plant volatile that, when applied to crops, has the potential to enhance natural enemy abundance and pest control. The impacts of MeSA in strawberry were unknown and examined in the spring and midsummer period. Strawberry plots contained no lures (control) or two 30-d MeSA lures (Predalure) in the center: one lure 0.61 m aboveground over a sticky trap, and one lure on a plant near the ground. Arthropod abundance was monitored at the point source, 5 m and 10 m away from lures over 31 d with white sticky traps, pitfall traps, and leaf inspection. Twenty-seven and nine comparisons were made among beneficial and pest arthropods, respectively. Overall positive responses were found among Chrysopidae in July-August 2008 and Orius tristicolor (White) in May-June 2009 to MeSA based on sticky traps. Chrysopidae showed attraction to the point source, but not at 5 m and 10 m. Ground-dwelling predators collected in pitfall traps such as Araneae, the carabid beetles, Pterostichus melanarius (Illiger), and Nebria brevicollis (Fabricius) did not respond. Increased abundance of six natural enemy groups appeared on various dates between 3 and 24 d after placement of lures in the field based on leaf inspection and sticky traps. Conversely, fewer Coccinellidae were captured on sticky traps on days 0-3, and fewer natural enemies were observed on leaves on day 28 in MeSA plots. MeSA did not increase nor decrease pest abundance.
Hair analysis as a useful procedure for detection of vapour exposure to chemical warfare agents: simulation of sulphur mustard with methyl salicylate.[Pubmed:24817050]
Drug Test Anal. 2014 Jun;6 Suppl 1:67-73.
Chemical warfare agents (CWA) are highly toxic compounds which have been produced to kill or hurt people during conflicts or terrorist attacks. Despite the fact that their use is strictly prohibited according to international convention, populations' exposure still recently occurred. Development of markers of exposure to CWA is necessary to distinguish exposed victims from unexposed ones. We present the first study of hair usage as passive sampler to assess contamination by chemicals in vapour form. This work presents more particularly the hair adsorption capacity for Methyl salicylate used as a surrogate of the vesicant sulphur mustard. Chemical vapours toxicity through the respiratory route has historically been defined through Haber's law's concentration-time (Ct) product, and vapour exposure of hair to Methyl salicylate was conducted with various times or doses of exposure in the range of incapacitating and lethal Ct products corresponding to sulphur mustard. Following exposure, extraction of Methyl salicylate from hair was conducted by simple soaking in dichloromethane. Methyl salicylate could be detected on hair for vapour concentration corresponding to about one fifth of the sulphur mustard concentration that would kill 50% of exposed individuals (LCt50). The amount of Methyl salicylate recovered from hair increased with time or dose of exposure. It showed a good correlation with the concentration-time product, suggesting that hair could be used like a passive sampler to assess vapour exposure to chemical compounds. It introduces great perspectives concerning the use of hair as a marker of exposure to CWA.
Plant methyl salicylate induces defense responses in the rhizobacterium Bacillus subtilis.[Pubmed:25181478]
Environ Microbiol. 2015 Apr;17(4):1365-76.
Bacillus subtilis is a rhizobacterium that promotes plant growth and health. Cultivation of B. subtilis with an uprooted weed on solid medium produced pleat-like architectures on colonies near the plant. To test whether plants emit signals that affect B. subtilis colony morphology, we examined the effect of plant-related compounds on colony morphology. Bacillus subtilis formed mucoid colonies specifically in response to Methyl salicylate, which is a plant-defense signal released in response to pathogen infection. Methyl salicylate induced mucoid colony formation by stimulating poly-gamma-glutamic acid biosynthesis, which formed enclosing capsules that protected the cells from exposure to antimicrobial compounds. Poly-gamma-glutamic acid synthesis depended on the DegS-DegU two-component regulatory system, which activated DegSU-dependent gene transcription in response to Methyl salicylate. Bacillus subtilis did not induce plant Methyl salicylate production, indicating that the most probable source of Methyl salicylate in the rhizosphere is pathogen-infected plants. Methyl salicylate induced B. subtilis biosynthesis of the antibiotics bacilysin and fengycin, the latter of which exhibited inhibitory activity against the plant pathogenic fungus Fusarium oxysporum. We propose that B. subtilis may sense plants under pathogen attack via Methyl salicylate, and express defense responses that protect both B. subtilis and host plants in the rhizosphere.
Fumigant Activity of 6 Selected Essential Oil Compounds and Combined Effect of Methyl Salicylate And Trans-Cinnamaldehyde Against Culex pipiens pallens.[Pubmed:25843095]
J Am Mosq Control Assoc. 2014 Sep;30(3):199-203.
We studied the knockdown activity and lethal toxicity of 6 essential oil compounds-Methyl salicylate, linalool, 2-phenethyl alcohol, eugenol, beta-citronellol, and trans-cinnamaldehyde-as fumigants against adult female Culex pipiens pallens in the laboratory. Of the 6 products tested, trans-cinnamaldehyde was the most toxic (LC50 = 0.26 microl/l air, 24 h) with a slow knockdown time (KT95 = 176.5 min at 0.5 microl/l air). Methyl salicylate displayed a lower toxicity (LC50 = 1.17 microl/l air, 24 h) but the fastest knockdown activity (KT95 = 16.8 min) at the sublethal concentration 0.5 microl/l air. Furthermore, the binary mixture of Methyl salicylate and trans-cinnamaldehyde exhibited a combined effect of fast knockdown activity and high toxicity against Cx. p. pallens adults, showing potential for development as natural fumigants for mosquito control.


