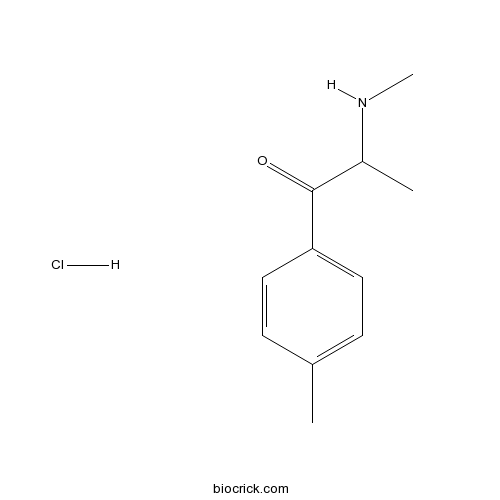
Mephedrone hydrochlorideInhibits synaptosomal dopamine and 5-HT uptake CAS# 1189726-22-4 |
- NQDI 1
Catalog No.:BCC2404
CAS No.:175026-96-7
- GRI 977143
Catalog No.:BCC2401
CAS No.:325850-81-5
- Mdivi 1
Catalog No.:BCC2402
CAS No.:338967-87-6
- DAPK Substrate Peptide
Catalog No.:BCC2400
CAS No.:386769-53-5
- Cesium chloride
Catalog No.:BCC2399
CAS No.:7647-17-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1189726-22-4 | SDF | Download SDF |
| PubChem ID | 46782120 | Appearance | Powder |
| Formula | C11H16ClNO | M.Wt | 213.7 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water and to 100 mM in DMSO | ||
| Chemical Name | 2-(methylamino)-1-(4-methylphenyl)propan-1-one;hydrochloride | ||
| SMILES | CC1=CC=C(C=C1)C(=O)C(C)NC.Cl | ||
| Standard InChIKey | DLQZFTUKJGRPLZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H15NO.ClH/c1-8-4-6-10(7-5-8)11(13)9(2)12-3;/h4-7,9,12H,1-3H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibits striatal dopamine uptake and hippocampal 5-HT uptake in synaptosomes (IC50 values are 467 and 558 nM respectively). Administration causes a decrease in dopamine and 5-HT transporter function. Synthetic CNS stimulant; brain penetrant. |

Mephedrone hydrochloride Dilution Calculator

Mephedrone hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.6795 mL | 23.3973 mL | 46.7946 mL | 93.5891 mL | 116.9864 mL |
| 5 mM | 0.9359 mL | 4.6795 mL | 9.3589 mL | 18.7178 mL | 23.3973 mL |
| 10 mM | 0.4679 mL | 2.3397 mL | 4.6795 mL | 9.3589 mL | 11.6986 mL |
| 50 mM | 0.0936 mL | 0.4679 mL | 0.9359 mL | 1.8718 mL | 2.3397 mL |
| 100 mM | 0.0468 mL | 0.234 mL | 0.4679 mL | 0.9359 mL | 1.1699 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ethyl ganoderate J
Catalog No.:BCN3486
CAS No.:1189555-95-0
- (S,S)-2,6-Bis(4-isopropyl-2-oxazolin-2-yl)pyridine
Catalog No.:BCC8402
CAS No.:118949-61-4
- 1,3-Dihydroxy-4-methoxy-10-methylacridin-9(10H)-one
Catalog No.:BCN1605
CAS No.:1189362-86-4
- 5-(3-Hydroxypropyl)-7-methoxybenzofuran
Catalog No.:BCN1606
CAS No.:118930-92-0
- Balanophonin
Catalog No.:BCN6072
CAS No.:118916-57-7
- Denudaquinol
Catalog No.:BCN8035
CAS No.:1189105-40-5
- Lettowienolide
Catalog No.:BCN8038
CAS No.:1189105-39-2
- Fmoc-D-Allo-Ile-OH
Catalog No.:BCC3508
CAS No.:118904-37-3
- Alstoyunine E
Catalog No.:BCN4782
CAS No.:1188932-15-1
- CEP-32496
Catalog No.:BCC1079
CAS No.:1188910-76-0
- KN-93 Phosphate
Catalog No.:BCC5638
CAS No.:1188890-41-6
- ABT 702 dihydrochloride
Catalog No.:BCC5905
CAS No.:1188890-28-9
- Fumitremorgin C
Catalog No.:BCC7507
CAS No.:118974-02-0
- 1-O-Deacetyl-2alpha-hydroxykhayanolide E
Catalog No.:BCN1604
CAS No.:1189801-51-1
- Viscumneoside III
Catalog No.:BCN7698
CAS No.:118985-27-6
- 8-Amino-2-naphthalenesulfonic acid
Catalog No.:BCC8783
CAS No.:119-28-8
- Methyl salicylate
Catalog No.:BCN5372
CAS No.:119-36-8
- 7-Anilino-4-hydroxy-2-naphthalenesulfonic acid
Catalog No.:BCC8777
CAS No.:119-40-4
- p-Anisoin
Catalog No.:BCC9113
CAS No.:119-52-8
- Benzoin
Catalog No.:BCC8854
CAS No.:119-53-9
- Benzophenone
Catalog No.:BCC8859
CAS No.:119-61-9
- 2-Carboxybenzaldehyde
Catalog No.:BCN2274
CAS No.:119-67-5
- 5-Amino-2-naphthalenesulfonic acid
Catalog No.:BCC8732
CAS No.:119-79-9
- 3,4-Dihydrocoumarin
Catalog No.:BCN6793
CAS No.:119-84-6
'Smoking' mephedrone: the identification of the pyrolysis products of 4-methylmethcathinone hydrochloride.[Pubmed:22641432]
Drug Test Anal. 2013 May;5(5):291-305.
The ring-substituted cathinone - mephedrone - has gained popularity among recreational drug users over the past several years. It is generally consumed orally or by snorting but reports indicate that it is also ingested by vaporization/inhalation. This study examines the pyrolysis products produced by heating mephedrone under using simulated 'meth pipe' conditions. Thirteen pyrolysis products were identified, the major ones being iso-mephedrone, 4-methylpropiophenone, 4-methylphenylacetone, two pyrazine derivatives formed by dimerization of mephedrone, N-methylated mephedrone (N,N,4-trimethylcatinone), two hydroxylated oxidation products and a diketone. Other minor products formed were identified as 4-methylacetophenone, two alpha-chloro ketones and N-methylated iso-mephedrone.
4-Methylmethcathinone (mephedrone): neuropharmacological effects of a designer stimulant of abuse.[Pubmed:21810934]
J Pharmacol Exp Ther. 2011 Nov;339(2):530-6.
The designer stimulant 4-methylmethcathinone (mephedrone) is among the most popular of the derivatives of the naturally occurring psychostimulant cathinone. Mephedrone has been readily available for legal purchase both online and in some stores and has been promoted by aggressive Web-based marketing. Its abuse in many countries, including the United States, is a serious public health concern. Owing largely to its recent emergence, there are no formal pharmacodynamic or pharmacokinetic studies of mephedrone. Accordingly, the purpose of this study was to evaluate effects of this agent in a rat model. Results revealed that, similar to methylenedioxymethamphetamine, methamphetamine, and methcathinone, repeated mephedrone injections (4x 10 or 25 mg/kg s.c. per injection, 2-h intervals, administered in a pattern used frequently to mimic psychostimulant "binge" treatment) cause a rapid decrease in striatal dopamine (DA) and hippocampal serotonin (5-hydroxytryptamine; 5HT) transporter function. Mephedrone also inhibited both synaptosomal DA and 5HT uptake. Like methylenedioxymethamphetamine, but unlike methamphetamine or methcathinone, repeated mephedrone administrations also caused persistent serotonergic, but not dopaminergic, deficits. However, mephedrone caused DA release from a striatal suspension approaching that of methamphetamine and was self-administered by rodents. A method was developed to assess mephedrone concentrations in rat brain and plasma, and mephedrone levels were determined 1 h after a binge treatment. These data demonstrate that mephedrone has a unique pharmacological profile with both abuse liability and neurotoxic potential.


