ABT 702 dihydrochlorideAdenosine kinase inhibitor CAS# 1188890-28-9 |
- ABT-199
Catalog No.:BCC3614
CAS No.:1257044-40-8
- BM-1074
Catalog No.:BCC2235
CAS No.:1391108-10-3
- HA14-1
Catalog No.:BCC3593
CAS No.:65673-63-4
- Obatoclax mesylate (GX15-070)
Catalog No.:BCC2234
CAS No.:803712-79-0
- ABT-737
Catalog No.:BCC3613
CAS No.:852808-04-9
- TW-37
Catalog No.:BCC2257
CAS No.:877877-35-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1188890-28-9 | SDF | Download SDF |
| PubChem ID | 16760265 | Appearance | Powder |
| Formula | C22H21BrCl2N6O | M.Wt | 536.26 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 33.33 mg/mL (62.15 mM) *"≥" means soluble, but saturation unknown. | ||
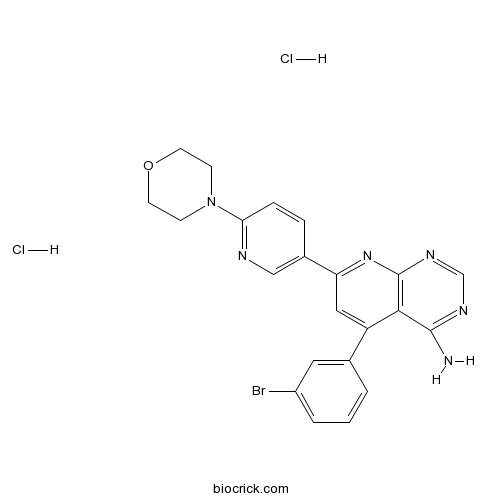
| Chemical Name | 5-(3-bromophenyl)-7-(6-morpholin-4-ylpyridin-3-yl)pyrido[2,3-d]pyrimidin-4-amine;dihydrochloride | ||
| SMILES | C1COCCN1C2=NC=C(C=C2)C3=NC4=C(C(=C3)C5=CC(=CC=C5)Br)C(=NC=N4)N.Cl.Cl | ||
| Standard InChIKey | OOXNYFKPOPJIOT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H19BrN6O.2ClH/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29;;/h1-5,10-13H,6-9H2,(H2,24,26,27,28);2*1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent non-nucleoside adenosine kinase inhibitor (IC50 = 1.7 nM), selective over other sites of adenosine interaction (A1, A2A and A3 receptors, adenosine transporter and adenosine deaminase). Displays oral activity in animal models of pain and inflammation. |

ABT 702 dihydrochloride Dilution Calculator

ABT 702 dihydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8648 mL | 9.3238 mL | 18.6477 mL | 37.2953 mL | 46.6192 mL |
| 5 mM | 0.373 mL | 1.8648 mL | 3.7295 mL | 7.4591 mL | 9.3238 mL |
| 10 mM | 0.1865 mL | 0.9324 mL | 1.8648 mL | 3.7295 mL | 4.6619 mL |
| 50 mM | 0.0373 mL | 0.1865 mL | 0.373 mL | 0.7459 mL | 0.9324 mL |
| 100 mM | 0.0186 mL | 0.0932 mL | 0.1865 mL | 0.373 mL | 0.4662 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: 1.7 nM for adenosine kinase
Adenosine (ADO) is an endogenous homeostatic inhibitory neuromodulator that reduces cellular excitability at sites of tissue injury and inflammation. Inhibition of adenosine kinase (AK), the primary metabolic enzyme for ADO, selectively increases ADO concentrations at sites of tissue trauma and enhances the analgesic and antiinflammatory actions of ADO. ABT 702 is a novel, potent nonnucleoside AK inhibitor.
In vitro: ABT 702 was active both in inhibiting AK (IC50 ) 1.7 nM) and ADO phosphorylation in the intact cells (IC50 ) 50 nM). ABT 702 was also highly selective for AK inhibition as compared to other sites of ADO action including ADA, ADO receptors, and ADO transport sites [1].
In vivo: ABT 702 had dose-dependent antinociceptive and antiinflammatory actions in a variety of animal models of nociceptive, inflammatory, and neuropathic pain. ABT 702 is the first of a novel class of potent, selective, non-nucleoside, orally active AK inhibitors that have potent antinociceptive effects in animal models [1].
Clinical trial: Up to now, ABT 702 is still in the preclinical development stage.
Reference:
[1] Lee CH, Jiang M, Cowart M, Gfesser G, Perner R, Kim KH, Gu YG, Williams M, Jarvis MF, Kowaluk EA, Stewart AO, Bhagwat SS. Discovery of 4-amino-5-(3-bromophenyl)-7-(6-morpholino-pyridin-3-yl)pyrido[2,3-d]pyrimidine, an orally active, non-nucleoside adenosine kinase inhibitor.. J Med Chem. 2001 Jun 21;44(13):2133-8.
- NBQX
Catalog No.:BCC6624
CAS No.:118876-58-7
- Anti-Inflammatory Peptide 1
Catalog No.:BCC1006
CAS No.:118850-71-8
- Ustusolate C
Catalog No.:BCN6755
CAS No.:1188398-15-3
- Ustusol C
Catalog No.:BCN6757
CAS No.:1188398-13-1
- 15,16-Di-O-acetyldarutoside
Catalog No.:BCN6071
CAS No.:1188282-02-1
- 16-O-Acetyldarutigenol
Catalog No.:BCN6070
CAS No.:1188282-01-0
- 9-Hydroxydarutigenol
Catalog No.:BCN6069
CAS No.:1188282-00-9
- 7-Hydroxydarutigenol
Catalog No.:BCN6068
CAS No.:1188281-99-3
- ent-14,16-Epoxy-8-pimarene-3,15-diol
Catalog No.:BCN6067
CAS No.:1188281-98-2
- Ac-Leu-OH
Catalog No.:BCC2967
CAS No.:1188-21-2
- Diosbulbin J
Catalog No.:BCN6066
CAS No.:1187951-06-9
- Diosbulbin I
Catalog No.:BCN6065
CAS No.:1187951-05-8
- KN-93 Phosphate
Catalog No.:BCC5638
CAS No.:1188890-41-6
- CEP-32496
Catalog No.:BCC1079
CAS No.:1188910-76-0
- Alstoyunine E
Catalog No.:BCN4782
CAS No.:1188932-15-1
- Fmoc-D-Allo-Ile-OH
Catalog No.:BCC3508
CAS No.:118904-37-3
- Lettowienolide
Catalog No.:BCN8038
CAS No.:1189105-39-2
- Denudaquinol
Catalog No.:BCN8035
CAS No.:1189105-40-5
- Balanophonin
Catalog No.:BCN6072
CAS No.:118916-57-7
- 5-(3-Hydroxypropyl)-7-methoxybenzofuran
Catalog No.:BCN1606
CAS No.:118930-92-0
- 1,3-Dihydroxy-4-methoxy-10-methylacridin-9(10H)-one
Catalog No.:BCN1605
CAS No.:1189362-86-4
- (S,S)-2,6-Bis(4-isopropyl-2-oxazolin-2-yl)pyridine
Catalog No.:BCC8402
CAS No.:118949-61-4
- Ethyl ganoderate J
Catalog No.:BCN3486
CAS No.:1189555-95-0
- Mephedrone hydrochloride
Catalog No.:BCC6183
CAS No.:1189726-22-4
Discovery of 4-amino-5-(3-bromophenyl)-7-(6-morpholino-pyridin-3-yl)pyrido[2,3-d]pyrimidine, an orally active, non-nucleoside adenosine kinase inhibitor..[Pubmed:11405650]
J Med Chem. 2001 Jun 21;44(13):2133-8.
Adenosine (ADO) is an endogenous homeostatic inhibitory neuromodulator that reduces cellular excitability at sites of tissue injury and inflammation. Inhibition of adenosine kinase (AK), the primary metabolic enzyme for ADO, selectively increases ADO concentrations at sites of tissue trauma and enhances the analgesic and antiinflammatory actions of ADO. Optimization of the high-throughput screening lead, 4-amino-7-aryl-substituted pteridine (5) (AK IC(50) = 440 nM), led to the identification of compound 21 (4-amino-5-(3-bromophenyl)-7-(6-morpholino-pyridin-3-yl)pyrido [2,3-d]pyrimidine, ABT-702), a novel, potent (AK IC(50) = 1.7 nM) non-nucleoside AK inhibitor with oral activity in animal models of pain and inflammation.
ABT-702 (4-amino-5-(3-bromophenyl)-7-(6-morpholinopyridin-3-yl)pyrido[2, 3-d]pyrimidine), a novel orally effective adenosine kinase inhibitor with analgesic and anti-inflammatory properties: I. In vitro characterization and acute antinociceptive effects in the mouse.[Pubmed:11082453]
J Pharmacol Exp Ther. 2000 Dec;295(3):1156-64.
Adenosine (ADO) is an inhibitory neuromodulator that can increase nociceptive thresholds in response to noxious stimulation. Inhibition of the ADO-metabolizing enzyme adenosine kinase (AK) increases extracellular ADO concentrations at sites of tissue trauma and AK inhibitors may have therapeutic potential as analgesic and anti-inflammatory agents. ABT-702 is a novel and potent (IC(50) = 1. 7 nM) non-nucleoside AK inhibitor that has several orders of magnitude selectivity over other sites of ADO interaction (A(1), A(2A), A(3) receptors, ADO transporter, and ADO deaminase). ABT-702 was 1300- to 7700-fold selective for AK compared with a number of other neurotransmitter and peptide receptors, ion channel proteins, neurotransmitter/nucleoside reuptake sites, and enzymes, including cycloxygenases-1 and -2. ABT-702 was equipotent (IC(50) = 1.5 +/- 0. 3 nM) in inhibiting native human AK (placenta), two human recombinant isoforms (AK(long) and AK(short)), and AK from monkey, dog, rat, and mouse brain. Kinetic studies revealed that AK inhibition by ABT-702 was competitive with respect to ADO and noncompetitive with respect to MgATP(2-). AK inhibition by ABT-702 was demonstrated to be reversible after 4 h of dialysis. ABT-702 is orally active and fully efficacious in reducing acute somatic nociception (ED(50) = 8 micromol/kg i.p.; 65 micromol/kg p.o.) in the mouse hot-plate assay. ABT-702 also dose dependently reduced nociception in the phenyl-p-quinone-induced abdominal constriction assay. The antinociceptive effects of ABT-702 in the hot-plate assay were blocked by the nonselective ADO receptor antagonist theophylline, and by the A(1)-selective antagonist cyclopentyltheophylline (10 mg/kg i.p.), but not by a peripherally selective ADO receptor antagonist 8-(p-sulfophenyl)-theophylline (50 mg/kg i.p.), by the A(2A)-selective antagonist 3, 7-dimethyl-1-propargylxanthine (1 mg/kg i.p.) or the opioid antagonist naloxone (5 mg/kg i.p.). Thus, ABT-702 is a novel and potent non-nucleoside AK inhibitor that effectively reduces acute thermal nociception in the mouse by a nonopioid, non-nonsteroidal anti-inflammatory drug, ADO A(1) receptor-mediated mechanism.
ABT-702 (4-amino-5-(3-bromophenyl)-7-(6-morpholino-pyridin- 3-yl)pyrido[2,3-d]pyrimidine), a novel orally effective adenosine kinase inhibitor with analgesic and anti-inflammatory properties. II. In vivo characterization in the rat.[Pubmed:11082454]
J Pharmacol Exp Ther. 2000 Dec;295(3):1165-74.
Adenosine kinase (AK; EC 2.7.1.20) is a key intracellular enzyme regulating intra-and extracellular concentrations of adenosine (ADO), an endogenous neuromodulator, antinociceptive, and anti-inflammatory autocoid. AK inhibition provides a means of potentiating local tissue concentrations of endogenous ADO, and AK inhibitors may have therapeutic potential as analgesic and anti-inflammatory agents. The effects of ABT-702, a novel, potent (IC(50) = 1.7 nM), and selective non-nucleoside AK inhibitor were examined in rat models of nociception and acute inflammation. ABT-702 was orally effective and fully efficacious to suppress nociception in a spectrum of pain models in the rat, including carrageenan-induced thermal hyperalgesia, the formalin test of persistent pain, and models of nerve injury-induced and diabetic neuropathic pain (tactile allodynia after L5/L6 spinal nerve ligation or streptozotocin injection, respectively.) ABT-702 was especially potent at relieving inflammatory thermal hyperalgesia (ED(50) = 5 micromol/kg p.o.). ABT-702 was also effective in the carrageenan-induced paw edema model of acute inflammation (ED(50) = 70 micromol/kg p.o.). The antinociceptive and anti-inflammatory effects of ABT-702 were blocked by selective ADO receptor antagonists, consistent with endogenous ADO accumulation and ADO receptor activation as a mechanism of action. The antinociceptive effects of ABT-702 were not blocked by the opioid antagonist naloxone. In addition, ABT-702 showed less potential to develop tolerance to its antinociceptive effects compared with morphine. ABT-702 had no significant effect on rotorod performance or heart rate (at 30-300 micromol/kg p.o.), mean arterial pressure (at 30-100 micromol/kg p.o.), or exploratory locomotor activity (at


